The RpoS Gatekeeper in Borrelia burgdorferi: An Invariant Regulatory Scheme That Promotes Spirochete Persistence in Reservoir Hosts and Niche Diversity
- PMID: 31507550
- PMCID: PMC6719511
- DOI: 10.3389/fmicb.2019.01923
The RpoS Gatekeeper in Borrelia burgdorferi: An Invariant Regulatory Scheme That Promotes Spirochete Persistence in Reservoir Hosts and Niche Diversity
Abstract
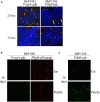
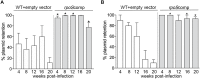
Maintenance of Borrelia burgdorferi within its enzootic cycle requires a complex regulatory pathway involving the alternative σ factors RpoN and RpoS and two ancillary trans-acting factors, BosR and Rrp2. Activation of this pathway occurs within ticks during the nymphal blood meal when RpoS, the effector σ factor, transcribes genes required for tick transmission and mammalian infection. RpoS also exerts a 'gatekeeper' function by repressing σ70-dependent tick phase genes (e.g., ospA, lp6.6). Herein, we undertook a broad examination of RpoS functionality throughout the enzootic cycle, beginning with modeling to confirm that this alternative σ factor is a 'genuine' RpoS homolog. Using a novel dual color reporter system, we established at the single spirochete level that ospA is expressed in nymphal midguts throughout transmission and is not downregulated until spirochetes have been transmitted to a naïve host. Although it is well established that rpoS/RpoS is expressed throughout infection, its requirement for persistent infection has not been demonstrated. Plasmid retention studies using a trans-complemented ΔrpoS mutant demonstrated that (i) RpoS is required for maximal fitness throughout the mammalian phase and (ii) RpoS represses tick phase genes until spirochetes are acquired by a naïve vector. By transposon mutant screening, we established that bba34/oppA5, the only OppA oligopeptide-binding protein controlled by RpoS, is a bona fide persistence gene. Lastly, comparison of the strain 297 and B31 RpoS DMC regulons identified two cohorts of RpoS-regulated genes. The first consists of highly conserved syntenic genes that are similarly regulated by RpoS in both strains and likely required for maintenance of B. burgdorferi sensu stricto strains in the wild. The second includes RpoS-regulated plasmid-encoded variable surface lipoproteins ospC, dbpA and members of the ospE/ospF/elp, mlp, revA, and Pfam54 paralogous gene families, all of which have evolved via inter- and intra-strain recombination. Thus, while the RpoN/RpoS pathway regulates a 'core' group of orthologous genes, diversity within RpoS regulons of different strains could be an important determinant of reservoir host range as well as spirochete virulence.
Keywords: Borrelia burgdorferi; Lyme disease; gene regulation; host adaptation; persistence; rpoS; sigma factor; vector borne disease.
Figures





References
-
- Arnold W. K., Savage C. R., Lethbridge K. G., Smith T. C., II, Brissette C. A., Seshu J., et al. (2018). Transcriptomic insights on the virulence-controlling CsrA, BadR, RpoN, and RpoS regulatory networks in the Lyme disease spirochete. PLoS One 13:e0203286. 10.1371/journal.pone.0203286 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

