The Impact of Infection in Pregnancy on Placental Vascular Development and Adverse Birth Outcomes
- PMID: 31507551
- PMCID: PMC6713994
- DOI: 10.3389/fmicb.2019.01924
The Impact of Infection in Pregnancy on Placental Vascular Development and Adverse Birth Outcomes
Abstract
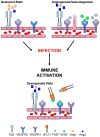
Healthy fetal development is dependent on nutrient and oxygen transfer via the placenta. Optimal growth and function of placental vasculature is therefore essential to support in utero development. Vasculogenesis, the de novo formation of blood vessels, and angiogenesis, the branching and remodeling of existing vasculature, mediate the development and maturation of placental villi, which form the materno-fetal interface. Several lines of evidence indicate that systemic maternal infection and consequent inflammation can disrupt placental vasculogenesis and angiogenesis. The resulting alterations in placental hemodynamics impact fetal growth and contribute to poor birth outcomes including preterm delivery, small-for-gestational age (SGA), stillbirth, and low birth weight (LBW). Furthermore, pathways involved in maternal immune activation and placental vascularization parallel those involved in normal fetal development, notably neurovascular development. Therefore, immune-mediated disruption of angiogenic pathways at the materno-fetal interface may also have long-term neurological consequences for offspring. Here, we review current literature evaluating the influence of maternal infection and immune activation at the materno-fetal interface and the subsequent impact on placental vascular function and birth outcome. Immunomodulatory pathways, including chemokines and cytokines released in response to maternal infection, interact closely with the principal pathways regulating placental vascular development, including the angiopoietin-Tie-2, vascular endothelial growth factor (VEGF), and placental growth factor (PlGF) pathways. A detailed mechanistic understanding of how maternal infections impact placental and fetal development is critical to the design of effective interventions to promote placental growth and function and thereby reduce adverse birth outcomes.
Keywords: adverse birth outcomes; infection; placenta; pregnancy; vascular development.
Figures

Similar articles
-
Early pregnancy maternal and fetal angiogenic factors and fetal and childhood growth: the Generation R Study.Hum Reprod. 2015 Jun;30(6):1302-13. doi: 10.1093/humrep/dev070. Epub 2015 Apr 8. Hum Reprod. 2015. PMID: 25854264
-
Inhibition of the C-X-C Motif Chemokine 12 (CXCL12) and Its Receptor CXCR4 Reduces Utero-Placental Expression of the VEGF System and Increases Utero-Placental Autophagy.Front Vet Sci. 2021 Aug 16;8:650687. doi: 10.3389/fvets.2021.650687. eCollection 2021. Front Vet Sci. 2021. PMID: 34485423 Free PMC article.
-
Pregnancy outcome and placental weights: their relationship to HIV-1 infection.East Afr Med J. 1993 Feb;70(2):85-9. East Afr Med J. 1993. PMID: 8513748
-
The impact of periconceptional maternal lifestyle on clinical features and biomarkers of placental development and function: a systematic review.Hum Reprod Update. 2019 Jan 1;25(1):72-94. doi: 10.1093/humupd/dmy037. Hum Reprod Update. 2019. PMID: 30407510
-
Oxygen and placental vascular development.Adv Exp Med Biol. 1999;474:259-75. doi: 10.1007/978-1-4615-4711-2_20. Adv Exp Med Biol. 1999. PMID: 10635006 Review.
Cited by
-
Associations of Plasmodium and Intestinal Helminth Infections with Maternal Anemia and Adverse Pregnancy Outcomes in Northwest Ethiopia.Am J Trop Med Hyg. 2024 Jul 9;111(3):498-505. doi: 10.4269/ajtmh.24-0080. Print 2024 Sep 4. Am J Trop Med Hyg. 2024. PMID: 38981502
-
Hematological Profile of Pregnant Women with Suspected Zika Virus Infection Followed Up at a Referral Service in Manaus, Brazil.Viruses. 2021 Apr 20;13(4):710. doi: 10.3390/v13040710. Viruses. 2021. PMID: 33923877 Free PMC article.
-
Pregnancy Outcomes in Women Screened for Tuberculosis Infection in Swedish Antenatal Care.Clin Infect Dis. 2024 Jan 25;78(1):125-132. doi: 10.1093/cid/ciad465. Clin Infect Dis. 2024. PMID: 37572363 Free PMC article.
-
Pre-conditioning with PQQ during pregnancy alleviates LPS-induced placental damage and improves the fetal survival and growth in mice.Front Endocrinol (Lausanne). 2025 Jul 10;16:1617026. doi: 10.3389/fendo.2025.1617026. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40708725 Free PMC article.
-
IFN-β reduces NRP-1 expression on human cord blood monocytes and inhibits VEGF-induced chemotaxis.Cytokine. 2021 Jul;143:155519. doi: 10.1016/j.cyto.2021.155519. Epub 2021 Apr 13. Cytokine. 2021. PMID: 33858750 Free PMC article.
References
-
- Ahmed A., Dunk C., Kniss D., Wilkes M. (1997). Role of VEGF receptor-1 (Flt-1) in mediating calcium-dependent nitric oxide release and limiting DNA synthesis in human trophoblast cells. Lab. Investig. 76, 779–791. PMID: - PubMed
-
- Ahmed A., Li X. F., Dunk C., Whittle M. J., Rushton D. I., Rollason T. (1995). Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors 12, 235–243. - PubMed
-
- Ahmed R., Singh N., ter Kuile F. O., Bharti P. K., Singh P. P., Desai M., et al. . (2014). Placental infections with histologically confirmed Plasmodium falciparum are associated with adverse birth outcomes in India: a cross-sectional study. Malar. J. 13:232. 10.1186/1475-2875-13-232, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

