Bacterial Profiling and Dynamic Succession Analysis of Phlebopus portentosus Casing Soil Using MiSeq Sequencing
- PMID: 31507552
- PMCID: PMC6716355
- DOI: 10.3389/fmicb.2019.01927
Bacterial Profiling and Dynamic Succession Analysis of Phlebopus portentosus Casing Soil Using MiSeq Sequencing
Abstract
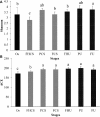
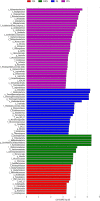
Phlebopus portentosus (Berk. and Broome) Boedijin is a popular edible mushroom found in China and Thailand. To date, P. portentosus is the only species in the order Boletales that can be successfully cultivated worldwide. The use of a casing layer or casing soil overlaying the substrate is a crucial step in the production of this mushroom. In this study, bacterial profiling and dynamic succession analyses of casing soil during the cultivation of P. portentosus were performed. One hundred and fifty samples were collected, and MiSeq sequencing of the V3-V4 region of the 16S rRNA gene was conducted. After performing a decontamination procedure, only 38 samples were retained, including 6 casing soil-originated samples (OS), 6 casing soil samples (FHCS) and 5 upper substrate samples (FHCU) from the period of complete colonization by mycelia; 6 casing soil samples (PCS) and 5 upper substrate samples (PCU) from the primordium period; and 6 casing soil samples (FCS) and 4 upper substrate samples (FCU) from fruit body period. The results revealed that bacterial diversity increased sharply from the hyphal to the primordium stage and then decreased during harvesting. The non-metric multidimensional scaling (NMDS) ordination and analysis of similarities (ANOSIM) analysis suggested that the community composition during different stages was significantly different in casing soil. The most abundant phyla in all of the samples were Proteobacteria, Chloroflexi, Acidobacteria, Actinobacteria, Saccharibacteria, and Bacteroidetes. Burkholderia was the most abundant genus in all the samples except the OS samples. The relative abundance of Burkholderia in the FHCS samples (55.79%) decreased to 35.14% in the PCS samples and then increased to 45.60% in the FCS samples. The abundances of Acidobacterium, Rhizobium, Acidisphaera, Bradyrhizobium, and Bacillus increased from the FHCS to PCS samples. The linear discriminant analysis (LDA) effect size (LEfSe) suggested that Acidobacterium and Acidisphaera are micromarkers for PCS, whereas Bradyrhizobium, Roseiarcus, and Pseudolabrys were associated with fruit body stages. The network analyses resulted in 23 edges, including 4 negative and 19 positive edges. Extensive mutualistic interactions may occur among casing soil bacteria. Furthermore, these bacteria play important roles in mycelial elongation, primordium formations, and the production of increased yields.
Keywords: MiSeq; bacterial diversity; casing soil; decontamination; dynamics.
Figures





Similar articles
-
Whole-Genome and Transcriptome Sequencing of Phlebopus portentosus Reveals Its Associated Ectomycorrhizal Niche and Conserved Pathways Involved in Fruiting Body Development.Front Microbiol. 2021 Sep 29;12:732458. doi: 10.3389/fmicb.2021.732458. eCollection 2021. Front Microbiol. 2021. PMID: 34659161 Free PMC article.
-
Casing microbiome dynamics during button mushroom cultivation: implications for dry and wet bubble diseases.Microbiology (Reading). 2019 Jun;165(6):611-624. doi: 10.1099/mic.0.000792. Epub 2019 Apr 17. Microbiology (Reading). 2019. PMID: 30994437
-
Influence of Agaricus bisporus establishment and fungicidal treatments on casing soil metataxonomy during mushroom cultivation.BMC Genomics. 2022 Jun 15;23(1):442. doi: 10.1186/s12864-022-08638-x. BMC Genomics. 2022. PMID: 35701764 Free PMC article.
-
Effect of Casing Layer on Growth Promotion of the Edible Mushroom Pleurotus ostreatus.Mycobiology. 2008 Mar;36(1):40-4. doi: 10.4489/MYCO.2008.36.1.040. Epub 2008 Mar 31. Mycobiology. 2008. PMID: 23997606 Free PMC article.
-
Hiseq Base Molecular Characterization of Soil Microbial Community, Diversity Structure, and Predictive Functional Profiling in Continuous Cucumber Planted Soil Affected by Diverse Cropping Systems in an Intensive Greenhouse Region of Northern China.Int J Mol Sci. 2019 May 28;20(11):2619. doi: 10.3390/ijms20112619. Int J Mol Sci. 2019. PMID: 31141960 Free PMC article.
Cited by
-
Whole-Genome and Transcriptome Sequencing of Phlebopus portentosus Reveals Its Associated Ectomycorrhizal Niche and Conserved Pathways Involved in Fruiting Body Development.Front Microbiol. 2021 Sep 29;12:732458. doi: 10.3389/fmicb.2021.732458. eCollection 2021. Front Microbiol. 2021. PMID: 34659161 Free PMC article.
-
Morel Production Associated with Soil Nitrogen-Fixing and Nitrifying Microorganisms.J Fungi (Basel). 2022 Mar 14;8(3):299. doi: 10.3390/jof8030299. J Fungi (Basel). 2022. PMID: 35330300 Free PMC article.
-
A preliminary study of the salivary microbiota of young male subjects before, during, and after acute high-altitude exposure.PeerJ. 2023 Jun 27;11:e15537. doi: 10.7717/peerj.15537. eCollection 2023. PeerJ. 2023. PMID: 37397022 Free PMC article.
-
Dynamics of soil microbiome throughout the cultivation life cycle of morel (Morchella sextelata).Front Microbiol. 2023 Feb 22;14:979835. doi: 10.3389/fmicb.2023.979835. eCollection 2023. Front Microbiol. 2023. PMID: 36910237 Free PMC article.
-
Method Superior to Traditional Spectral Identification: FT-NIR Two-Dimensional Correlation Spectroscopy Combined with Deep Learning to Identify the Shelf Life of Fresh Phlebopus portentosus.ACS Omega. 2021 Jul 22;6(30):19665-19674. doi: 10.1021/acsomega.1c02317. eCollection 2021 Aug 3. ACS Omega. 2021. PMID: 34368554 Free PMC article.
References
-
- Bandala V. M., Montoya L., Jarvio D. (2004). Two interesting records of Boletes found in coffee plantations in eastern Mexico. Persoonia 18, 365–380.
-
- Carrasco J., Tello M. L., Perez M., Preston G. (2019b). Biotechnological requirements for the commercial cultivation of macrofungi: substrate and casing layer. Biol. Macrofungi 159–175. 10.1007/978-3-030-02622-6_7 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

