Implication of Viral Infections for Greenhouse Gas Dynamics in Freshwater Wetlands: Challenges and Perspectives
- PMID: 31507569
- PMCID: PMC6718870
- DOI: 10.3389/fmicb.2019.01962
Implication of Viral Infections for Greenhouse Gas Dynamics in Freshwater Wetlands: Challenges and Perspectives
Abstract
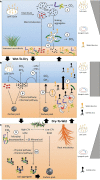
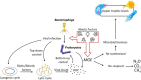
Viruses are non-living, acellular entities, and the most abundant biological agents on earth. They are widely acknowledged as having the capacity to influence global biogeochemical cycles by infecting the bacterial and archaeal populations that regulate carbon and nutrient turnover. Evidence suggests that the majority of viruses in wetlands are bacteriophages, but despite their importance, studies on how viruses control the prokaryotic community and the concomitant impacts on ecosystem function (such as carbon cycling and greenhouse gas flux) in wetlands are rare. Here we investigate virus-prokaryote interactions in freshwater wetland ecosystems in the context of their potential influence on biogeochemical cycling. Specifically, we (1) synthesize existing literature to establish current understanding of virus-prokaryote interactions, focusing on the implications for wetland greenhouse gas dynamics and (2) identify future research priorities. Viral dynamics in freshwater wetlands have received much less attention compared to those in marine ecosystems. However, based on our literature review, within the last 10 years, viral ecology studies on freshwater wetlands have increased twofold. Despite this increase in literature, the potential implication of viral infections on greenhouse gas emission dynamics is still a knowledge gap. We hypothesize that the rate of greenhouse gas emissions and the pool of sequestered carbon could be strongly linked to the type and rate of viral infection. Viral replication mechanism choice will consequently influence the microbial efficiency of organic matter assimilation and thus the ultimate fate of carbon as a greenhouse gas or stored in soils.
Keywords: biogeochemical cycles; carbon dioxide; freshwater; infection; methane; prokaryote; virus; wetland.
Figures

Similar articles
-
The potential of viruses to influence the magnitude of greenhouse gas emissions in an inland wetland.Water Res. 2021 Apr 1;193:116875. doi: 10.1016/j.watres.2021.116875. Epub 2021 Jan 27. Water Res. 2021. PMID: 33550166
-
Patterns in wetland microbial community composition and functional gene repertoire associated with methane emissions.mBio. 2015 May 19;6(3):e00066-15. doi: 10.1128/mBio.00066-15. mBio. 2015. PMID: 25991679 Free PMC article.
-
The role of environmental driving factors in historical and projected carbon dynamics of wetland ecosystems in Alaska.Ecol Appl. 2018 Sep;28(6):1377-1395. doi: 10.1002/eap.1755. Epub 2018 Jun 25. Ecol Appl. 2018. PMID: 29808543
-
Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales.Glob Chang Biol. 2013 May;19(5):1325-46. doi: 10.1111/gcb.12131. Epub 2013 Feb 11. Glob Chang Biol. 2013. PMID: 23505021 Review.
-
Carbon Cycling in Wetlands Under the Shadow of Microplastics: Challenges and Prospects.Toxics. 2025 Feb 20;13(3):143. doi: 10.3390/toxics13030143. Toxics. 2025. PMID: 40137470 Free PMC article. Review.
Cited by
-
Virus and Potential Host Microbes from Viral-Enriched Metagenomic Characterization in the High-Altitude Wetland, Salar de Huasco, Chile.Microorganisms. 2020 Jul 20;8(7):1077. doi: 10.3390/microorganisms8071077. Microorganisms. 2020. PMID: 32698305 Free PMC article.
-
Top-Down Controls of Bacterial Metabolism: A Case Study from a Temperate Freshwater Lake Ecosystem.Microorganisms. 2022 Mar 25;10(4):715. doi: 10.3390/microorganisms10040715. Microorganisms. 2022. PMID: 35456766 Free PMC article.
-
The "Regulator" Function of Viruses on Ecosystem Carbon Cycling in the Anthropocene.Front Public Health. 2022 Mar 29;10:858615. doi: 10.3389/fpubh.2022.858615. eCollection 2022. Front Public Health. 2022. PMID: 35425734 Free PMC article.
-
DNase Treatment Improves Viral Enrichment in Agricultural Soil Viromes.mSystems. 2021 Oct 26;6(5):e0061421. doi: 10.1128/mSystems.00614-21. Epub 2021 Sep 7. mSystems. 2021. PMID: 34491084 Free PMC article.
References
-
- Ackermann H. W., DuBow M. S. (eds.) (1987). “Viruses of prokaryotes” in General Properties of Bacteriophages. Vol. 1 (Boca Raton, CA, USA: CRC Press; ), 202.
-
- Alexyuk V., Alexyuk M., Bogoyavlenskiy P., Komarnytsky A., Mirzadinov S., Zhumanov R., et al. (2016). Diversity of viral photosystem-II psb A genes in the largest channel reservoir of Kazakhstan. Res. J. Pharm. Biol. Chem. Sci. 7, 2674–2680. ISSN: 09758585.
-
- Azam F., Fenchel T., Field J. G., Gray J. S., Meyer-Reil L. A., Thingstad F. (1983). The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263. 10.3354/meps010257 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

