ADAM17-Mediated Reduction in CD14++CD16+ Monocytes ex vivo and Reduction in Intermediate Monocytes With Immune Paresis in Acute Pancreatitis and Acute Alcoholic Hepatitis
- PMID: 31507587
- PMCID: PMC6718469
- DOI: 10.3389/fimmu.2019.01902
ADAM17-Mediated Reduction in CD14++CD16+ Monocytes ex vivo and Reduction in Intermediate Monocytes With Immune Paresis in Acute Pancreatitis and Acute Alcoholic Hepatitis
Abstract
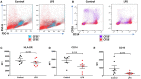
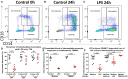
Impaired immune responses and increased susceptibility to infection characterize acute inflammatory conditions such as pancreatitis and alcoholic hepatitis and are major causes of morbidity and mortality. However, the mechanisms that drive this apparent immune paresis remain poorly understood. Monocytes mediate host responses to damage and pathogens in health and disease, and three subsets of monocytes have been defined based on CD14 and CD16 expression. We sought to determine the changes in monocyte subsets in acute pancreatitis (AP) and acute alcoholic hepatitis (AAH), together with functional consequences and mechanisms that underlie this change. Peripheral blood mononuclear cells (PBMCs) from patients with AP or AAH were compared with healthy controls. Monocyte subsets were defined by HLA-DR, CD14, and CD16 expression. Changes in surface and intracellular protein expression and phosphorylation were determined by flow cytometry. Phenotype and function were assessed following stimulation with lipopolysaccharide (LPS) or other agonists in the presence of specific inhibitors of TNFα and a disintegrin and metalloproteinase 17 (ADAM17). Patients with AP and AAH had reduced CD14++CD16+ intermediate monocytes compared to controls. Reduction of intermediate monocytes was recapitulated ex vivo by stimulating healthy control PBMCs with Toll-like receptor (TLR) agonists LPS, flagellin or polyinosilic:polycytidylic acid (poly I:C). Stimulation caused shedding of CD14 and CD16, which could be reversed using the ADAM17 inhibitor, TMI005 but not direct inhibitors of TNFα, a known ADAM17-target. Culturing PBMCs from healthy controls resulted in expansion of intermediate monocytes, which did not occur when LPS was in the culture medium. Cultured intermediate monocytes showed reduced expression of CX3CR1, CCR2, TLR4, and TLR5. We found reduced migratory responses, intracellular signaling and pro-inflammatory cytokine production, and increased expression of IL-10. Stimulation with TLR agonists results in ADAM17-mediated shedding of phenotypic markers from CD16+ monocytes, leading to apparent "loss" of intermediate monocytes. Reduction in CD14++CD16- monocytes and increased CD14++CD16+ is associated with altered responses in functional assays ex vivo. Patients with AP and AAH had reduced proportions of CD14++CD16+ monocytes and reduced phosphorylation of NFκB and IL-6 production in response to bacterial LPS. Together, these processes may contribute to the susceptibility to infection observed in AP and AAH.
Keywords: ADAM17; acute alcoholic hepatitis; acute pancreatitis; infection; inflammation; monocytes.
Figures





Similar articles
-
Intermediate Monocytes in Acute Alcoholic Hepatitis Are Functionally Activated and Induce IL-17 Expression in CD4+ T Cells.J Immunol. 2019 Dec 15;203(12):3190-3198. doi: 10.4049/jimmunol.1800742. Epub 2019 Nov 13. J Immunol. 2019. PMID: 31722987
-
A Novel, Five-Marker Alternative to CD16-CD14 Gating to Identify the Three Human Monocyte Subsets.Front Immunol. 2019 Jul 26;10:1761. doi: 10.3389/fimmu.2019.01761. eCollection 2019. Front Immunol. 2019. PMID: 31402918 Free PMC article.
-
Acute Alcohol Intoxication Modulates Monocyte Subsets and Their Functions in a Time-Dependent Manner in Healthy Volunteers.Front Immunol. 2021 May 18;12:652488. doi: 10.3389/fimmu.2021.652488. eCollection 2021. Front Immunol. 2021. PMID: 34084163 Free PMC article.
-
Maximal Exercise Alters the Inflammatory Phenotype and Response of Mononuclear Cells.Med Sci Sports Exerc. 2018 Apr;50(4):675-683. doi: 10.1249/MSS.0000000000001480. Med Sci Sports Exerc. 2018. PMID: 29112629
-
Signalling pathways in alcohol-induced liver inflammation.J Hepatol. 2009 Jun;50(6):1258-66. doi: 10.1016/j.jhep.2009.03.007. Epub 2009 Mar 28. J Hepatol. 2009. PMID: 19398236 Free PMC article. Review.
Cited by
-
Helminth extracellular vesicles co-opt host monocytes to drive T cell anergy.J Extracell Vesicles. 2025 Jan;14(1):e70027. doi: 10.1002/jev2.70027. J Extracell Vesicles. 2025. PMID: 39815783 Free PMC article.
-
Immunoprofiling of monocytes in STAT1 gain-of-function chronic mucocutaneous candidiasis.Front Immunol. 2022 Sep 12;13:983977. doi: 10.3389/fimmu.2022.983977. eCollection 2022. Front Immunol. 2022. PMID: 36172362 Free PMC article.
-
Immune profiles of a COVID-19 adolescent with mild symptoms and anti-viral antibody deficiency.Fundam Res. 2021 Mar;1(2):117-123. doi: 10.1016/j.fmre.2021.02.004. Epub 2021 Feb 17. Fundam Res. 2021. PMID: 40477411 Free PMC article. No abstract available.
-
An early regulatory mechanism of hyperinflammation by restricting monocyte contribution.Front Immunol. 2024 Jul 8;15:1398153. doi: 10.3389/fimmu.2024.1398153. eCollection 2024. Front Immunol. 2024. PMID: 39040105 Free PMC article.
-
ADAM17-Mediated Shedding of Inflammatory Cytokines in Hypertension.Front Pharmacol. 2020 Jul 29;11:1154. doi: 10.3389/fphar.2020.01154. eCollection 2020. Front Pharmacol. 2020. PMID: 32848763 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

