Metabolic Control of Innate Lymphoid Cell Migration
- PMID: 31507605
- PMCID: PMC6713999
- DOI: 10.3389/fimmu.2019.02010
Metabolic Control of Innate Lymphoid Cell Migration
Abstract
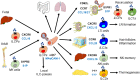
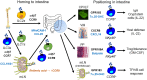
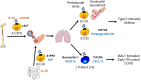
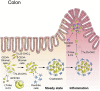
Innate lymphoid cells (ILCs) are specialized immune cells that rapidly respond to environmental challenges, such as infection and tissue damage. ILCs play an important role in organ homeostasis, tissue repair, and host defense in the mucosal tissues intestine and lung. ILCs are sentinels of healthy tissue function, yet it is poorly understood how ILCs are recruited, strategically positioned, and maintained within tissues. Accordingly, ILC migration is an area that has recently come into focus and it is important to define the signals that control ILC migration to and within tissues. In this context, signals from the local tissue microenvironment are relevant. For example, ILCs in the intestine are exposed to an environment that is rich in dietary, microbial, and endogenous metabolites. It has been shown that the Vitamin A metabolite retinoic acid promotes ILC1 and ILC3 homing to the intestine. In addition, recent studies have discovered cholesterol metabolites (oxysterols) as a novel class of molecules that regulate ILC migration through the receptor GPR183. ILCs are considered to be largely tissue-resident cells, yet recent data indicate that ILCs actively migrate during inflammation. Furthermore, the discovery of circulating ILC precursors in humans and their presence within tissues has fueled the concept of local ILC-poiesis. However, it is unclear how circulating ILCs enter tissue during embryogenesis and inflammation and how they are directed to local tissue niches. In this review, I will discuss the metabolic signals that regulate ILC homing and their strategic positioning in healthy and inflamed tissues. It is becoming increasingly clear that ILC function is closely linked to their tissue localization. Therefore, understanding the tissue signals that control ILC migration could open new avenues for the treatment of chronic inflammatory diseases and cancer.
Keywords: cancer; inflammation; innate lymphoid cells; metabolism; migration; oxysterol.
Figures
Similar articles
-
Cytokine Networks between Innate Lymphoid Cells and Myeloid Cells.Front Immunol. 2018 Feb 7;9:191. doi: 10.3389/fimmu.2018.00191. eCollection 2018. Front Immunol. 2018. PMID: 29467768 Free PMC article. Review.
-
Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut.Immunity. 2015 Jul 21;43(1):107-19. doi: 10.1016/j.immuni.2015.06.009. Epub 2015 Jun 30. Immunity. 2015. PMID: 26141583 Free PMC article.
-
Local and systemic features of ILC immunometabolism.Curr Opin Hematol. 2022 Jul 1;29(4):209-217. doi: 10.1097/MOH.0000000000000722. Curr Opin Hematol. 2022. PMID: 35787549 Review.
-
ILC-poiesis: Ensuring tissue ILC differentiation at the right place and time.Eur J Immunol. 2019 Jan;49(1):11-18. doi: 10.1002/eji.201747294. Epub 2018 Nov 2. Eur J Immunol. 2019. PMID: 30350853 Review.
-
Metabolism of Natural Killer Cells and Other Innate Lymphoid Cells.Front Immunol. 2020 Aug 28;11:1989. doi: 10.3389/fimmu.2020.01989. eCollection 2020. Front Immunol. 2020. PMID: 32983138 Free PMC article. Review.
Cited by
-
Protective and pathogenic functions of innate lymphoid cells in transplantation.Clin Exp Immunol. 2023 Jul 5;213(1):23-39. doi: 10.1093/cei/uxad050. Clin Exp Immunol. 2023. PMID: 37119279 Free PMC article. Review.
-
The Impacts of Cholesterol, Oxysterols, and Cholesterol Lowering Dietary Compounds on the Immune System.Int J Mol Sci. 2022 Oct 13;23(20):12236. doi: 10.3390/ijms232012236. Int J Mol Sci. 2022. PMID: 36293093 Free PMC article. Review.
-
Majie Cataplasm Promotes Th1 Response to Fight against Asthmatic Th2 Inflammation through NKs.Evid Based Complement Alternat Med. 2022 May 12;2022:6745420. doi: 10.1155/2022/6745420. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35600943 Free PMC article.
-
Single-cell atlas of the small intestine throughout the human lifespan demonstrates unique features of fetal immune cells.Mucosal Immunol. 2024 Aug;17(4):599-617. doi: 10.1016/j.mucimm.2024.03.011. Epub 2024 Mar 28. Mucosal Immunol. 2024. PMID: 38555026 Free PMC article.
-
Tissue-Specific Metabolic Reprogramming in Innate Lymphoid Cells and Its Impact on Disease.Immune Netw. 2025 Feb 7;25(1):e3. doi: 10.4110/in.2025.25.e3. eCollection 2025 Feb. Immune Netw. 2025. PMID: 40078781 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

