Interleukin-1/-33 Signaling Pathways as Therapeutic Targets for Endometriosis
- PMID: 31507610
- PMCID: PMC6714064
- DOI: 10.3389/fimmu.2019.02021
Interleukin-1/-33 Signaling Pathways as Therapeutic Targets for Endometriosis
Abstract
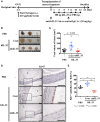
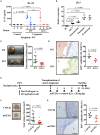
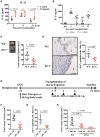
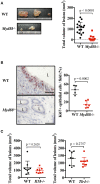
Endometriosis is an estrogen-dependent disease with symptoms of dysmenorrhea, chronic pain, and infertility that affects 6-10% of women of reproductive age. Medical or surgical therapy, such as administration of an anti-gonadotropin or ovarian cystectomy, provide effective pain relief. However, neither therapy can be used for patients wishing to become pregnant. Despite the high morbidity, the pathogenesis of endometriosis has not been well-elucidated. Several inflammatory cytokines are reported to participate in the onset of endometriosis. Here, we examined the role of interleukin (IL)-1/IL-33 signaling in the development of endometriosis using a mouse model of endometriosis. Endometriotic lesion volume was significantly reduced in Il33-/- and Il1r1-/- mice, and almost completely suppressed in Myd88-/- mice. Mice intraperitoneally administered with an antibody against IL-1 receptor 1 (IL-1R1) or IL-33 developed limited endometriotic lesions. Oral administration of an inhibitor against IL-1R-associated kinase 4 (IRAK4), a downstream signal molecule of MyD88, also suppressed lesion formation. Furthermore, even after the development of cystic lesions the IRAK4 inhibitor prevented the enlargement of lesions. These treatments all significantly reduced cellular proliferation, shown by decreased Ki-67 expression. These results reveal that IL-1/IL-1R1, IL-33/IL-33R and associated downstream signaling molecules are involved in the pathogenesis of endometriosis, and may provide novel therapeutic targets for endometriosis.
Keywords: IL-1R1; IRAK4; MyD88; ST2; estrogen.
Figures
Similar articles
-
Interleukin-33 modulates inflammation in endometriosis.Sci Rep. 2017 Dec 20;7(1):17903. doi: 10.1038/s41598-017-18224-x. Sci Rep. 2017. PMID: 29263351 Free PMC article.
-
Postoperative ileus involves interleukin-1 receptor signaling in enteric glia.Gastroenterology. 2014 Jan;146(1):176-87.e1. doi: 10.1053/j.gastro.2013.09.030. Epub 2013 Sep 22. Gastroenterology. 2014. PMID: 24067878
-
Thymol Impacts the Progression of Endometriosis by Disrupting Estrogen Signaling Pathways and Inflammatory Responses.Int J Mol Sci. 2024 Dec 7;25(23):13150. doi: 10.3390/ijms252313150. Int J Mol Sci. 2024. PMID: 39684860 Free PMC article.
-
Celecoxib attenuates interleukin 33-induced expression of vascular cell adhesion molecule-1 in human ovarian endometriotic stromal cells.Taiwan J Obstet Gynecol. 2024 Mar;63(2):178-185. doi: 10.1016/j.tjog.2024.01.012. Taiwan J Obstet Gynecol. 2024. PMID: 38485312 Review.
-
Dysfunctional signaling underlying endometriosis: current state of knowledge.J Mol Endocrinol. 2018 Apr;60(3):R97-R113. doi: 10.1530/JME-17-0227. Epub 2018 Jan 12. J Mol Endocrinol. 2018. PMID: 29330150 Review.
Cited by
-
Long noncoding RNA ADAMTS9-AS1 represses ferroptosis of endometrial stromal cells by regulating the miR-6516-5p/GPX4 axis in endometriosis.Sci Rep. 2022 Feb 16;12(1):2618. doi: 10.1038/s41598-022-04963-z. Sci Rep. 2022. PMID: 35173188 Free PMC article.
-
A Lifelong Impact on Endometriosis: Pathophysiology and Pharmacological Treatment.Int J Mol Sci. 2023 Apr 19;24(8):7503. doi: 10.3390/ijms24087503. Int J Mol Sci. 2023. PMID: 37108664 Free PMC article. Review.
-
Research advances in drug therapy of endometriosis.Front Pharmacol. 2023 Jun 21;14:1199010. doi: 10.3389/fphar.2023.1199010. eCollection 2023. Front Pharmacol. 2023. PMID: 37416064 Free PMC article. Review.
-
The coagulation status in women of endometriosis with stage IV.BMC Womens Health. 2024 Jul 4;24(1):386. doi: 10.1186/s12905-024-03227-4. BMC Womens Health. 2024. PMID: 38961373 Free PMC article.
-
Pyroptotic T cell-derived active IL-16 has a driving function in ovarian endometriosis development.Cell Rep Med. 2024 Mar 19;5(3):101476. doi: 10.1016/j.xcrm.2024.101476. Cell Rep Med. 2024. PMID: 38508138 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

