Subcutaneous Immunoglobulin Twenty Percent Every Two Weeks in Pediatric Patients with Primary Immunodeficiencies: Subcohort Analysis of the IBIS Study
- PMID: 31508259
- PMCID: PMC6733055
- DOI: 10.1089/ped.2018.0967
Subcutaneous Immunoglobulin Twenty Percent Every Two Weeks in Pediatric Patients with Primary Immunodeficiencies: Subcohort Analysis of the IBIS Study
Abstract
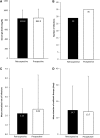
Background: Subcutaneous immunoglobulin G (SCIG) may be a better option than intravenous immunoglobulin G (IVIG) for patients with primary immunodeficiencies (PID) due to reduced systemic and serious adverse reactions and easier administration. The Infusione Bimensile di Immunoglobuline Sottocute (IBIS) study investigated the effects of Hizentra®, a 20%-concentrated SCIG, administered biweekly in patients with PID. This subanalysis aimed to evaluate clinical and laboratory outcomes in the IBIS pediatric subcohort. Methods: Thirteen children with PID were observed for 12 months retrospectively (with previous IVIG/SCIG) and prospectively with biweekly Hizentra. Results: Mean ± standard deviation serum IG levels during the retrospective (833.8 ± 175.7 mg/dL) and the prospective (842.0 ± 188.0 mg/dL) phases were comparable; there were also no differences in the number of infections. Conclusions: Biweekly Hizentra is a noninferior option with respect to previous IVIG/SCIG-based treatment.
Keywords: children; immunoglobulin; pediatric; primary immunodeficiencies; subcutaneous.
Conflict of interest statement
G.M.B. is an employee of CSL Behring. C.C., V.G., C.P., A.T., S.G., B.M., V.M., V.P., A.M., A.P., G.S., A.V., and C.A. declare no conflicts of interest.
Figures

References
-
- Buckley RH. Primary immunodeficiency diseases due to defects in lymphocytes. N Engl J Med 2000; 343:1313–1324 - PubMed
-
- Radinsky S, Bonagura VR. Subcutaneous immunoglobulin infusion as an alternative to intravenous immunoglobulin. J Allergy Clin Immunol 2003; 112:630–633 - PubMed
-
- Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies-a prospective, multi-national study. J Clin Immunol 2006; 26:177–185 - PubMed
LinkOut - more resources
Full Text Sources

