Construction of Hierarchical Co-Fe Oxyphosphide Microtubes for Electrocatalytic Overall Water Splitting
- PMID: 31508276
- PMCID: PMC6724352
- DOI: 10.1002/advs.201900576
Construction of Hierarchical Co-Fe Oxyphosphide Microtubes for Electrocatalytic Overall Water Splitting
Abstract
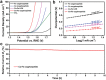
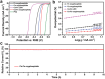
Development of efficient electrocatalysts is a crucial requirement to build water splitting systems for the production of clean and sustainable fuels. This goal could be achieved by fine-tuning the composition and structure of the electrocatalytic materials. Here, a facile self-templated synthetic strategy is developed for the fabrication of hierarchical Co-Fe oxyphosphide microtubes (MTs). Fe-based metal-organic compound microrods are first synthesized as the self-sacrificing template. Afterward, the Fe-based precursors are converted into hierarchical Co-Fe layered double hydroxide MTs through a hydrothermal approach, which are then transformed into the hierarchical Co-Fe oxyphosphide MTs by a phosphidation treatment. Benefiting from the synergistic effect of the compositions and the advantages of the hierarchical hollow structure, the obtained electrocatalyst exhibits enhanced performance for overall water splitting.
Keywords: hierarchical; hollow; microtubes; oxyphosphides; water splitting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
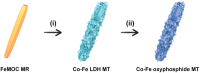


References
-
- Mallouk T. E., Nat. Chem. 2013, 5, 362. - PubMed
-
- Turner J. A., Science 2004, 305, 972. - PubMed
-
- Montoya J. H., Seitz L. C., Chakthranont P., Vojvodic A., Jaramillo T. F., Nørskov J. K., Nat. Mater. 2017, 16, 70. - PubMed
-
- Bi W., Zhang L., Sun Z., Li X., Jin T., Wu X., Zhang Q., Luo Y., Wu C., Xie Y., ACS Catal. 2016, 6, 4253.
LinkOut - more resources
Full Text Sources
Other Literature Sources
