Cytomembrane-Mediated Transport of Metal Ions with Biological Specificity
- PMID: 31508286
- PMCID: PMC6724363
- DOI: 10.1002/advs.201900835
Cytomembrane-Mediated Transport of Metal Ions with Biological Specificity
Abstract
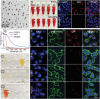
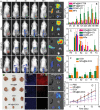
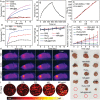
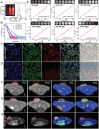
Metal ions are of significant importance in biomedical science. This study reports a new concept of cytomembrane-mediated biospecific transport of metal ions without using any other materials. For the first time, cytomembranes are exploited for two-step conjugation with metal ions to provide hybrid nanomaterials. The innate biofunction of cell membranes renders the hybrids with superior advantages over common vehicles for metal ions, including excellent biocompatibility, low immunogenic risk, and particularly specific biotargeting functionality. As a proof-of-concept demonstration, cancer cell membranes are used for in vivo delivery of various metal ions, including ruthenium, europium, iron, and manganese, providing a series of tumor-targeted nanohybrids capable of photothermal therapy/imaging, magnetic resonance imaging, photoacoustic imaging, and fluorescence imaging with improved performances. In addition, the special structure of the cell membrane allows easy accommodation of small-molecular agents within the nanohybrids for effective chemotherapy. This study provides a new class of metal-ion-included nanomaterials with versatile biofunctions and offers a novel solution to address the important challenge in the field of in vivo targeted delivery of metal ions.
Keywords: bioimaging; biotargeted transport; cell membrane; metal ions; tumor therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Guo Z., Sadler P. J., Angew. Chem., Int. Ed. 1999, 38, 1512. - PubMed
-
- a) Taylor‐Pashow K. M. L., Della Rocca J., Xie Z., Tran S., Lin W., J. Am. Chem. Soc. 2009, 131, 14261; - PMC - PubMed
- b) Horcajada P., Serre C., Maurin G., Ramsahye N. A., Balas F., Vallet‐Regí M., Sebban M., Taulelle F., Férey G., J. Am. Chem. Soc. 2008, 130, 6774; - PubMed
- c) Sun C.‐Y., Qin C., Wang C.‐G., Su Z.‐M., Wang S., Wang X.‐L., Yang G.‐S., Shao K.‐Z., Lan Y.‐Q., Wang E.‐B., Adv. Mater. 2011, 23, 5629. - PubMed
-
- a) Bornscheuer U. T., Kazlauskas R. J., Angew. Chem., Int. Ed. 2004, 43, 6032; - PubMed
- b) Steitz T. A., Steitz J. A., Proc. Natl. Acad. Sci. USA 1993, 90, 6498; - PMC - PubMed
- c) Khan M. T., Martell A. E., J. Am. Chem. Soc. 1967, 89, 4176; - PubMed
- d) Stadtman E. R., Free Radic. Biol. Med. 1990, 9, 315. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
