Material-Induced Venosome-Supported Bone Tubes
- PMID: 31508287
- PMCID: PMC6724474
- DOI: 10.1002/advs.201900844
Material-Induced Venosome-Supported Bone Tubes
Abstract
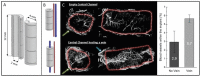
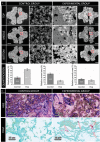
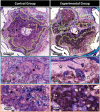
The development of alternatives to vascular bone grafts, the current clinical standard for the surgical repair of large segmental bone defects still today represents an unmet medical need. The subcutaneous formation of transplantable bone has been successfully achieved in scaffolds axially perfused by an arteriovenous loop (AVL) and seeded with bone marrow stromal cells or loaded with inductive proteins. Although demonstrating clinical potential, AVL-based approaches involve complex microsurgical techniques and thus are not in widespread use. In this study, 3D-printed microporous bioceramics, loaded with autologous total bone marrow obtained by needle aspiration, are placed around and next to an unoperated femoral vein for 8 weeks to assess the effect of a central flow-through vein on bone formation from marrow in a subcutaneous site. A greater volume of new bone tissue is observed in scaffolds perfused by a central vein compared with the nonperfused negative control. These analyses are confirmed and supplemented by calcified and decalcified histology. This is highly significant as it indicates that transplantable vascularized bone can be grown using dispensable vein and marrow tissue only. This is the first report illustrating the capacity of an intrinsic vascularization by a single vein to support ectopic bone formation from untreated marrow.
Keywords: angiogenesis; axial vascularization; bioceramic; bioinorganic; material–host interactions; osteogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Human Umbilical Vein Endothelial Cell Support Bone Formation of Adipose-Derived Stem Cell-Loaded and 3D-Printed Osteogenic Matrices in the Arteriovenous Loop Model.Tissue Eng Part A. 2021 Mar;27(5-6):413-423. doi: 10.1089/ten.TEA.2020.0087. Epub 2020 Sep 18. Tissue Eng Part A. 2021. PMID: 32723066
-
A tissue engineered 3D printed calcium alkali phosphate bioceramic bone graft enables vascularization and regeneration of critical-size discontinuity bony defects in vivo.Front Bioeng Biotechnol. 2023 Jun 15;11:1221314. doi: 10.3389/fbioe.2023.1221314. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37397960 Free PMC article.
-
In situ production of pre-vascularized synthetic bone grafts for regenerating critical-sized defects in rabbits.Acta Biomater. 2020 Sep 15;114:384-394. doi: 10.1016/j.actbio.2020.07.030. Epub 2020 Jul 18. Acta Biomater. 2020. PMID: 32688088
-
The Arteriovenous Loop: Engineering of Axially Vascularized Tissue.Eur Surg Res. 2018;59(3-4):286-299. doi: 10.1159/000492417. Epub 2018 Sep 21. Eur Surg Res. 2018. PMID: 30244238 Review.
-
Mechanism and application of 3D-printed degradable bioceramic scaffolds for bone repair.Biomater Sci. 2023 Oct 24;11(21):7034-7050. doi: 10.1039/d3bm01214j. Biomater Sci. 2023. PMID: 37782081 Review.
Cited by
-
A Composite Lactide-Mineral 3D-Printed Scaffold for Bone Repair and Regeneration.Front Cell Dev Biol. 2021 Jul 9;9:654518. doi: 10.3389/fcell.2021.654518. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34307346 Free PMC article.
-
[Research progress of in vivo bioreactor for bone tissue engineering].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021 May 15;35(5):627-635. doi: 10.7507/1002-1892.202012083. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021. Retraction in: Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022 Jan 15;36(1):9. doi: 10.7507/1002-1892.202200001. PMID: 33998218 Free PMC article. Retracted. Review. Chinese.
-
Intelligent Vascularized 3D/4D/5D/6D-Printed Tissue Scaffolds.Nanomicro Lett. 2023 Oct 31;15(1):239. doi: 10.1007/s40820-023-01187-2. Nanomicro Lett. 2023. PMID: 37907770 Free PMC article. Review.
-
3D Biomimetic Calcified Cartilaginous Callus that Induces Type H Vessels Formation and Osteoclastogenesis.Adv Sci (Weinh). 2023 Jun;10(16):e2207089. doi: 10.1002/advs.202207089. Epub 2023 Mar 31. Adv Sci (Weinh). 2023. PMID: 36999832 Free PMC article.
-
Recent advancement in vascularized tissue-engineered bone based on materials design and modification.Mater Today Bio. 2023 Nov 11;23:100858. doi: 10.1016/j.mtbio.2023.100858. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 38024843 Free PMC article. Review.
References
-
- Taylor G. I., Miller G. D., Ham F. J., Plast. Reconstr. Surg. 1975, 55, 533. - PubMed
-
- Pogrel M. A., Podlesh S., Anthony J. P., Alexander J., J. Oral Maxillofac. Surg. 1997, 55, 1200. - PubMed
-
- Hori Y., Tamai S., Okuda H., Sakamoto H., Takita T., Masuhara K., J. Hand Surg. 1979, 4, 23. - PubMed
-
- Tanaka Y., Sung K.‐C., Tsutsumi A., Ohba S., Ueda K., Morrison W. A., Plast. Reconstr. Surg. 2003, 112, 1636. - PubMed
-
- Erol O. O., Spira M., Plast. Reconstr. Surg. 1980, 66, 109. - PubMed
LinkOut - more resources
Full Text Sources
