Giant cell tumour of the bone treated with denosumab: How has the blood supply and oncological prognosis of the tumour changed?
- PMID: 31508313
- PMCID: PMC6718948
- DOI: 10.1016/j.jot.2018.10.003
Giant cell tumour of the bone treated with denosumab: How has the blood supply and oncological prognosis of the tumour changed?
Abstract
Background: Denosumab is gradually applied to refractory or unresectable giant cell tumour of the bone. Whether denosumab can effectively reduce the blood supply of tumour and bring benefit is worthy of study. The aim of the study is to evaluate the related changes after treatment: blood supply, surgical plan downstaging, surgical difficulty and oncological prognosis.
Methods: A self-case-control study was performed from June 2014 to November 2016, and 18 patients were enrolled. Patients received subcutaneous denosumab 120 mg every 4 weeks preoperatively, with additional doses administered on Days 8 and 15 during the first month of therapy. The initial treatment duration was 12 weeks. After 12 weeks treatment, enhanced CT examination was performed for evaluating whether surgical treatment was practicable. The patients received preoperative denosumab treatment for 5 (median 3, range 3-12) months in average. The microvessel density of tumour samples was calculated for evaluating tumour blood supply. The computed tomography (CT) enhancement rate was compared before and after treatment. The related changes of parameters were recorded as the following: clinical benefits, serious side effects, enhancement rate of CT, surgical plans, intraoperative blood loss, operative time, surgical difficulty, histological changes and local recurrence. The patients were followed up every 3 months postoperatively.
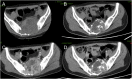
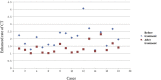
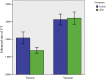
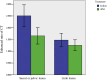
Results: The average CT enhancement rate of lesions was 2.08 and 1.40 before and after treatment (p = 0.000), respectively. The unenhanced CT value was significantly increased after treatment (p = 0.038). The CT enhancement rate changed more significantly in pelvic or sacral lesions than that in limb lesions (p = 0.024). Sixteen cases underwent final surgery, and surgical plan was downstaged. The histological examination showed tumour cells were significantly reduced or even disappeared after treatment. The microvessel density decreased significantly after treatment. The mean postoperative follow-up was 18.8 (10-31) months, and five patients had local recurrence. The high local recurrence rate (4/6) in sacral tumours may be related to the increased difficulty of curettage.
Conclusion: Denosumab treatment can reduce the blood supply of giant cell tumour. The sacral or pelvic lesions changed more significantly than limb lesions. The surgical plan downstaging can also be achieved. The clear margin after denosumab treatment facilitated tumour resection but, increased difficult in curettage surgery, and high recurrence rate of sacral tumour is being concerned.
The translational impact of this article: Denosumab is a new type of humanized monoclonal antibody which showed some effect in the treatment giant cell tumor of bone. Pre-operative treatment with denosamub can reduce intra-operative blood loss and down-stage surgical plan in suitable cases.
Keywords: Blood supply; Denosumab; Giant cell tumour of bone; Prognosis; Surgical treatment.
Figures



Similar articles
-
Outcome of re-operation for local recurrence following pre-operative denosumab administration and curettage for giant cell tumour of bone with difficult joint preservation.Int Orthop. 2023 Jan;47(1):265-273. doi: 10.1007/s00264-022-05613-9. Epub 2022 Oct 25. Int Orthop. 2023. PMID: 36282294
-
A nonrandomized controlled study of sacral giant cell tumors with preoperative treatment of denosumab.Medicine (Baltimore). 2018 Nov;97(46):e13139. doi: 10.1097/MD.0000000000013139. Medicine (Baltimore). 2018. PMID: 30431583 Free PMC article.
-
Study of imaging changes following preoperative denosumab for giant cell tumor of bone.J Bone Oncol. 2021 Dec 31;32:100410. doi: 10.1016/j.jbo.2021.100410. eCollection 2022 Feb. J Bone Oncol. 2021. PMID: 35059284 Free PMC article.
-
Giant cell tumour of bone in the denosumab era.Eur J Cancer. 2017 May;77:75-83. doi: 10.1016/j.ejca.2017.02.021. Epub 2017 Mar 30. Eur J Cancer. 2017. PMID: 28365529 Review.
-
The Role of Denosumab for Surgical Outcomes in Patients with Giant Cell Tumour of Bone: A Systematic Review.Curr Oncol. 2021 Mar 22;28(2):1302-1313. doi: 10.3390/curroncol28020124. Curr Oncol. 2021. PMID: 33809979 Free PMC article.
Cited by
-
Outcome of Reoperation for Local Recurrence Following En Bloc Resection for Bone Giant Cell Tumor of the Extremity.Curr Oncol. 2022 Sep 5;29(9):6383-6399. doi: 10.3390/curroncol29090503. Curr Oncol. 2022. PMID: 36135072 Free PMC article.
-
Personalized medicine modality based on patient-derived xenografts from a malignant transformed GCTB harboring H3F3A G34W mutation.J Orthop Translat. 2021 Jun 1;29:106-112. doi: 10.1016/j.jot.2021.04.004. eCollection 2021 Jul. J Orthop Translat. 2021. PMID: 34136349 Free PMC article.
-
The Effectiveness of Denosumab in Middle Eastern Patients With Giant Cell Tumor of the Bone: A Single-Center, Retrospective Study.Cureus. 2024 Apr 15;16(4):e58292. doi: 10.7759/cureus.58292. eCollection 2024 Apr. Cureus. 2024. PMID: 38752067 Free PMC article.
-
CT delta-radiomics predicts the risks of blood transfusion and massive bleeding during spinal tumor surgery.Cancer Imaging. 2025 Jun 22;25(1):79. doi: 10.1186/s40644-025-00900-1. Cancer Imaging. 2025. PMID: 40545537 Free PMC article.
-
Primary Cooperative Application of a LARS® Tube and 3D-Printed Prosthesis for Reconstruction of the Distal Radius after en bloc Resection of Giant Cell Tumor of Bone: A Comparative Retrospective Study.Orthop Surg. 2023 Jun;15(6):1521-1533. doi: 10.1111/os.13722. Epub 2023 Apr 20. Orthop Surg. 2023. PMID: 37078245 Free PMC article.
References
-
- Unni K.K., Inwards C.Y. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. Dahlin's bone tumors: general aspects and data on 10,165 cases.
-
- Campanacci M. 2nd ed. Springer; New York: 1999. Bone and soft tissue tumors: clinical features, imaging, pathology and treatment.
-
- Atkinson J., Cranmer P., Saunders T. AMG 162, a fully human RANK-L antibody, increases bone mass and bone strength in cynomolgus monkeys. J Bone Miner Res. 2005;20:S29.
LinkOut - more resources
Full Text Sources
Research Materials

