The Possibility of Deep Learning-Based, Computer-Aided Skin Tumor Classifiers
- PMID: 31508420
- PMCID: PMC6719629
- DOI: 10.3389/fmed.2019.00191
The Possibility of Deep Learning-Based, Computer-Aided Skin Tumor Classifiers
Abstract
The incidence of skin tumors has steadily increased. Although most are benign and do not affect survival, some of the more malignant skin tumors present a lethal threat if a delay in diagnosis permits them to become advanced. Ideally, an inspection by an expert dermatologist would accurately detect malignant skin tumors in the early stage; however, it is not practical for every single patient to receive intensive screening by dermatologists. To overcome this issue, many studies are ongoing to develop dermatologist-level, computer-aided diagnostics. Whereas, many systems that can classify dermoscopic images at this dermatologist-equivalent level have been reported, a much fewer number of systems that can classify conventional clinical images have been reported thus far. Recently, the introduction of deep-learning technology, a method that automatically extracts a set of representative features for further classification has dramatically improved classification efficacy. This new technology has the potential to improve the computer classification accuracy of conventional clinical images to the level of skilled dermatologists. In this review, this new technology and present development of computer-aided skin tumor classifiers will be summarized.
Keywords: artificial intelligence; clinical image; convolutional neural network; deep learning; dermoscopy; skin tumor classifier.
Figures


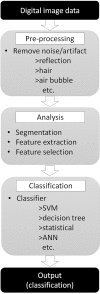
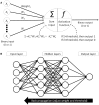
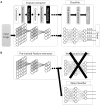

Similar articles
-
Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis.Br J Dermatol. 2019 Feb;180(2):373-381. doi: 10.1111/bjd.16924. Epub 2018 Sep 19. Br J Dermatol. 2019. PMID: 29953582
-
A convolutional neural network trained with dermoscopic images performed on par with 145 dermatologists in a clinical melanoma image classification task.Eur J Cancer. 2019 Apr;111:148-154. doi: 10.1016/j.ejca.2019.02.005. Epub 2019 Mar 8. Eur J Cancer. 2019. PMID: 30852421
-
Deep learning-based, computer-aided classifier developed with dermoscopic images shows comparable performance to 164 dermatologists in cutaneous disease diagnosis in the Chinese population.Chin Med J (Engl). 2020 Sep 5;133(17):2027-2036. doi: 10.1097/CM9.0000000000001023. Chin Med J (Engl). 2020. PMID: 32826613 Free PMC article.
-
Skin cancer classification via convolutional neural networks: systematic review of studies involving human experts.Eur J Cancer. 2021 Oct;156:202-216. doi: 10.1016/j.ejca.2021.06.049. Epub 2021 Sep 8. Eur J Cancer. 2021. PMID: 34509059
-
Deep Learning Approaches Towards Skin Lesion Segmentation and Classification from Dermoscopic Images - A Review.Curr Med Imaging. 2020;16(5):513-533. doi: 10.2174/1573405615666190129120449. Curr Med Imaging. 2020. PMID: 32484086 Review.
Cited by
-
In-Person Verification of Deep Learning Algorithm for Diabetic Retinopathy Screening Using Different Techniques Across Fundus Image Devices.Transl Vis Sci Technol. 2021 Nov 1;10(13):17. doi: 10.1167/tvst.10.13.17. Transl Vis Sci Technol. 2021. PMID: 34767624 Free PMC article.
-
A shallow deep learning approach to classify skin cancer using down-scaling method to minimize time and space complexity.PLoS One. 2022 Aug 4;17(8):e0269826. doi: 10.1371/journal.pone.0269826. eCollection 2022. PLoS One. 2022. PMID: 35925956 Free PMC article.
-
Next-Generation Technologies in Dermatology: Use of Artificial Intelligence and Mobile Applications.Indian J Dermatol. 2020 Sep-Oct;65(5):351. doi: 10.4103/ijd.IJD_433_20. Indian J Dermatol. 2020. PMID: 33165416 Free PMC article. No abstract available.
-
Impact of fine-tuning parameters of convolutional neural network for skin cancer detection.Sci Rep. 2025 Apr 28;15(1):14779. doi: 10.1038/s41598-025-99529-0. Sci Rep. 2025. PMID: 40295678 Free PMC article.
-
Agreement Between Experts and an Untrained Crowd for Identifying Dermoscopic Features Using a Gamified App: Reader Feasibility Study.JMIR Med Inform. 2023 Jan 18;11:e38412. doi: 10.2196/38412. JMIR Med Inform. 2023. PMID: 36652282 Free PMC article.
References
-
- United States Cancer Statistics: Data Visualiztions. Available online at: https://gis.cdc.gov/Cancer/USCS/DataViz.html (accessed May 28, 2019).
-
- Gruber SB, Armstrong BK. Cutaneous and ocular melanoma. In: Schottenfeld D, Fraumeni JF. editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; (2006). p. 1196–229. 10.1093/acprof:oso/9780195149616.003.0063 - DOI
Publication types
LinkOut - more resources
Full Text Sources

