Valve-in-Valve Challenges: How to Avoid Coronary Obstruction
- PMID: 31508426
- PMCID: PMC6716332
- DOI: 10.3389/fcvm.2019.00120
Valve-in-Valve Challenges: How to Avoid Coronary Obstruction
Abstract
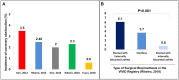
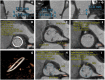
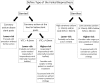
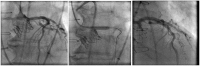
Coronary obstruction is a rare but life-threatening complication in patients undergoing transcatheter aortic valve replacement (TAVR). Aortic valve-in-valve (VIV) procedures to treat failed surgical bioprosthesis is associated with ~6-fold higher risk for coronary obstruction in certain situations. The primary mechanism consists in the occlusion of the coronary ostium by the dislodged leaflet from the bioprosthesis after deployment of the transcatheter heart valve (THV), which most commonly occurs during the index procedure, but in up to 1/3 of cases a delayed presentation ensues. The clinical presentation consists of severe hypotension and ECG changes in most of the patients, with very high mortality rates. Therefore, pre-procedural multi-slice computed tomography is crucial for identifying high-risk features, such as low coronary heights, shallow sinuses of Valsalva, and short virtual THV to coronary ostial distance (VTC). Also, some models of surgical bioprosthesis present an increased risk for this dreadful complication. Preemptive protective strategies with coronary wiring, with or without placement of an undeployed stent, could mitigate the risks associated with this complication in high-risk patients, even though studies are lacking. This review aims to take a clinical perspective on the challenges in avoiding this complication during VIV procedures.
Keywords: coronary obstruction; failed surgical bioprosthesis; transcatheter aortic valve replacement; transcatheter heart valve; valve-in-valve.
Figures


References
-
- Barbanti M, Webb JG, Tamburino C, Van Mieghem NM, Makkar RR, Piazza N, et al. . Outcomes of redo transcatheter aortic valve replacement for the treatment of postprocedural and late occurrence of paravalvular regurgitation and transcatheter valve failure. Circ Cardiovasc Interv. (2016) 9:1–10. 10.1161/CIRCINTERVENTIONS.116.003930 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

