Mouse transient receptor potential channel type 6 selectively regulates agonist-induced platelet function
- PMID: 31508510
- PMCID: PMC6726914
- DOI: 10.1016/j.bbrep.2019.100685
Mouse transient receptor potential channel type 6 selectively regulates agonist-induced platelet function
Abstract
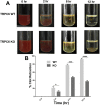
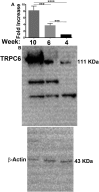
While changes in intracellular calcium levels is a central step in platelet activation and thrombus formation, the contribution and mechanism of receptor-operated calcium entry (ROCE) via transient receptor potential channels (TRPCs) in platelets remains poorly defined. In previous studies, we have shown that TRPC6 regulates hemostasis and thrombosis, in mice. In the present studies, we employed a knockout mouse model system to characterize the role of TRPC6 in ROCE and platelet activation. It was observed that the TRPC6 deletion (Trpc6 -/- ) platelets displayed impaired elevation of intracellular calcium, i.e., defective ROCE. Moreover, these platelets also exhibited defects in a host of functional responses, namely aggregation, granule secretion, and integrin αIIbβ3. Interestingly, the aforementioned defects were specific to the thromboxane receptor (TPR), as no impaired responses were observed in response to ADP or the thrombin receptor-activating peptide 4 (TRAP4). The defect in ROCE in the Trpc6 -/- was also observed with 1-oleoyl-2-acetyl-sn-glycerol (OAG). Finally, our studies also revealed that TRPC6 regulates clot retraction. Taken together, our findings demonstrate that TRPC6 directly regulates TPR-dependent ROCE and platelet function. Thus, TRPC6 may serve as a novel target for the therapeutic management of thrombotic diseases.
Keywords: Calcium channel; Platelets; Receptor-operated calcium entry; TRPC6.
Figures


Similar articles
-
A critical role for the transient receptor potential channel type 6 in human platelet activation.PLoS One. 2015 Apr 30;10(4):e0125764. doi: 10.1371/journal.pone.0125764. eCollection 2015. PLoS One. 2015. PMID: 25928636 Free PMC article.
-
Defective diacylglycerol-induced Ca2+ entry but normal agonist-induced activation responses in TRPC6-deficient mouse platelets.J Thromb Haemost. 2012 Mar;10(3):419-29. doi: 10.1111/j.1538-7836.2011.04596.x. J Thromb Haemost. 2012. PMID: 22176814
-
The canonical transient receptor potential 6 (TRPC6) channel is sensitive to extracellular pH in mouse platelets.Blood Cells Mol Dis. 2014 Feb-Mar;52(2-3):108-15. doi: 10.1016/j.bcmd.2013.08.007. Epub 2013 Sep 25. Blood Cells Mol Dis. 2014. PMID: 24074949
-
Mouse transient receptor potential channel 6: role in hemostasis and thrombogenesis.Biochem Biophys Res Commun. 2012 Jan 13;417(2):853-6. doi: 10.1016/j.bbrc.2011.12.058. Epub 2011 Dec 20. Biochem Biophys Res Commun. 2012. PMID: 22206677
-
Regulation of Platelet Function by Orai, STIM and TRP.Adv Exp Med Biol. 2016;898:157-81. doi: 10.1007/978-3-319-26974-0_8. Adv Exp Med Biol. 2016. PMID: 27161229 Review.
Cited by
-
Calcium: More Than Bone? Implications for Clinical Practice and Theory.J Clin Med Res. 2021 May;13(5):253-257. doi: 10.14740/jocmr4505. Epub 2021 May 25. J Clin Med Res. 2021. PMID: 34104276 Free PMC article. Review.
-
Mechanisms Underlying Dichotomous Procoagulant COAT Platelet Generation-A Conceptual Review Summarizing Current Knowledge.Int J Mol Sci. 2022 Feb 25;23(5):2536. doi: 10.3390/ijms23052536. Int J Mol Sci. 2022. PMID: 35269679 Free PMC article. Review.
-
A Putative Role for TRPC6 in Immune-Mediated Kidney Injury.Int J Mol Sci. 2023 Nov 16;24(22):16419. doi: 10.3390/ijms242216419. Int J Mol Sci. 2023. PMID: 38003608 Free PMC article. Review.
-
Trpc6 gain-of-function disease mutation enhances phosphatidylserine exposure in murine platelets.PLoS One. 2022 Jun 24;17(6):e0270431. doi: 10.1371/journal.pone.0270431. eCollection 2022. PLoS One. 2022. PMID: 35749414 Free PMC article.
-
Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke.Int J Mol Sci. 2021 Feb 19;22(4):2074. doi: 10.3390/ijms22042074. Int J Mol Sci. 2021. PMID: 33669830 Free PMC article. Review.
References
-
- Blockmans D., Deckmyn H., Vermylen J. Platelet activation. Blood Rev. 1995;9:143–156. - PubMed
-
- Varga-Szabo D., Authi K.S., Braun A., Bender M., Ambily A., Hassock S.R., Gudermann T., Dietrich A., Nieswandt B. Store-operated Ca(2+) entry in platelets occurs independently of transient receptor potential (TRP) C1. Pflüg. Arch. 2008;457:377–387. - PubMed
-
- Ramanathan G., Gupta S., Thielmann I., Pleines I., Varga-Szabo D., May F., Mannhalter C., Dietrich A., Nieswandt B., Braun A. Defective diacylglycerol-induced Ca2+ entry but normal agonist-induced activation responses in TRPC6-deficient mouse platelets. J. Thromb. Haemost. 2012;10:419–429. - PubMed
-
- Varga-Szabo D., Braun A., Nieswandt B. Calcium signaling in platelets. J. Thromb. Haemost. 2009;7:1057–1066. - PubMed
-
- Zhang Y., Pertens E., Janssen L.J. 8-isoprostaglandin E(2) activates Ca(2+)-dependent K(+) current via cyclic AMP signaling pathway in murine renal artery. Eur. J. Pharmacol. 2005;520:22–28. - PubMed
LinkOut - more resources
Full Text Sources

