Improved Bioavailability of Curcumin in Gliadin-Protected Gold Quantum Cluster for Targeted Delivery
- PMID: 31508538
- PMCID: PMC6732771
- DOI: 10.1021/acsomega.9b00917
Improved Bioavailability of Curcumin in Gliadin-Protected Gold Quantum Cluster for Targeted Delivery
Abstract
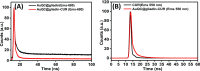
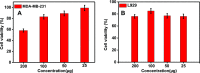
This study deals with the synthesis of a gliadin-stabilized gold quantum cluster (AuQC) for the encapsulation of curcumin (CUR) and its targeted delivery to the cancer cell. CUR is an anticancer drug containing a hydrophobic polyphenol derived from the rhizome of Curcuma longa. The utilization of CUR in cancer treatment is limited because of suboptimal pharmacokinetics and poor bioavailability at the tumor site. In order to improve the bioavailability of CUR, we have encapsulated it into AuQCs stabilized by a proline-rich protein gliadin because proline-rich protein has the ability to bind a hydrophobic drug CUR. The encapsulation of CUR into the hydrophobic cavity of the protein was confirmed by various spectroscopic techniques. Compared to CUR alone, the encapsulated CUR was stable against degradation and showed higher pH stability up to pH 8.5. The encapsulation efficiency of CUR in AuQCs was calculated as 98%, which was much higher than the other reported methods. In vitro drug release experiment exhibited a controlled and pH-dependent CUR release over a period of 60 h. The encapsulated CUR-QCs exhibited less toxicity in the normal cell line (L929) and high toxicity in breast cancer (MDA-MB239). Thus, it can be used as a potential material for anticancer therapy and bioimaging.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
LinkOut - more resources
Full Text Sources

