Evaluation of hydroxyurea genotoxicity in patients with sickle cell disease
- PMID: 31508660
- PMCID: PMC6750882
- DOI: 10.31744/einstein_journal/2019AO4742
Evaluation of hydroxyurea genotoxicity in patients with sickle cell disease
Abstract
Objective: To evaluate the induction of DNA damage in peripheral blood mononuclear cells of patients with sickle cell disease, SS and SC genotypes, treated with hydroxyurea.
Methods: The study subjects were divided into two groups: one group of 22 patients with sickle cell disease, SS and SC genotypes, treated with hydroxyurea, and a Control Group composed of 24 patients with sickle cell disease who were not treated with hydroxyurea. Peripheral blood samples were submitted to peripheral blood mononuclear cell isolation to assess genotoxicity by the cytokinesis-block micronucleus cytome assay, in which DNA damage biomarkers - micronuclei, nucleoplasmic bridges and nuclear buds - were counted.
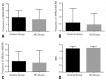
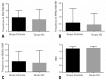
Results: Patients with sickle cell disease treated with hydroxyurea had a mean age of 25.4 years, whereas patients with sickle cell disease not treated with hydroxyurea had a mean age of 17.6 years. The mean dose of hydroxyurea used by the patients was 12.8mg/kg/day, for a mean period of 44 months. The mean micronucleus frequency per 1,000 cells of 8.591±1.568 was observed in the Hydroxyurea Group and 10.040±1.003 in the Control Group. The mean frequency of nucleoplasmic bridges per 1,000 cells and nuclear buds per 1,000 cells for the hydroxyurea and Control Groups were 0.4545±0.1707 versus 0.5833±0.2078, and 0.8182±0.2430 versus 0.9583±0.1853, respectively. There was no statistically significant difference between groups.
Conclusion: In the study population, patients with sickle cell disease treated with the standard dose of hydroxyurea treatment did not show evidence of DNA damage induction.
Objetivo: Avaliar o efeito da indução de danos ao DNA em células monocelulares do sangue periférico de pacientes com doença falciforme, genótipos SS e SC, tratados com hidroxiureia.
Métodos: Os sujeitos da pesquisa foram divididos em dois grupos: um de 22 pacientes com doença falciforme genótipos SS e SC tratados com hidroxiureia, e o outro controle, composto por 24 pacientes com doença falciforme que não eram tratados com o fármaco. As amostras de sangue periférico foram submetidas ao isolamento de células mononucleares do sangue periférico para avaliação da genotoxicidade pelo ensaio de micronúcleo citoma com bloqueio da citocinese, tendo sido quantificados os biomarcadores de danos ao DNA – micronúcleos, pontes nucleoplasmáticas e brotamento nuclear.
Resultados: Os pacientes com doença falciforme tratados com hidroxiureia apresentaram média de idade de 25,4 anos, enquanto aqueles com doença falciforme não tratados com hidroxiureia tiveram média de idade de 17,6 anos. A dose média de hidroxiureia utilizada pelos pacientes foi de 12,8mg/kg/dia, por período médio de 44 meses. A frequência média de micronúcleos por 1.000 células de 8,591±1,568 foi observada no Grupo Hidroxiureia e de 10,040±1,003 no Grupo Controle. Adicionalmente, a frequência média de pontes nucleoplasmáticas por 1.000 células e brotamento nuclear por 1.000 células para o Grupo Hidroxiureia e Controle foi de 0,4545±0,1707 versus 0,5833±0,2078, e de 0,8182±0,2430 versus 0,9583±0,1853, respectivamente. Não houve diferença estatisticamente significativa entre os grupos.
Conclusão: Na população estudada de pacientes com doença falciforme com tratamento em dose padrão de hidroxiureia, não houve evidência de indução de danos ao DNA.
Conflict of interest statement
Conflict of interest:
none.
Figures
References
-
- 3. Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev. 2017;4:CD002202. Review. - PMC - PubMed
- Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev. 2017;4:CD002202. Review. - PMC - PubMed
-
- 5. Strouse JJ, Lanzkron S, Beach MC, Haywood C, Park H, Witkop C, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008;122(6):1332-42. Review. - PubMed
- Strouse JJ, Lanzkron S, Beach MC, Haywood C, Park H, Witkop C, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008;122(6):1332–1342. Review. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

