Discovery of retinoic acid receptor agonists as proliferators of cardiac progenitor cells through a phenotypic screening approach
- PMID: 31508905
- PMCID: PMC6954720
- DOI: 10.1002/sctm.19-0069
Discovery of retinoic acid receptor agonists as proliferators of cardiac progenitor cells through a phenotypic screening approach
Abstract
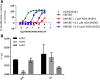
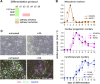
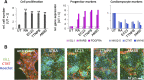
Identification of small molecules with the potential to selectively proliferate cardiac progenitor cells (CPCs) will aid our understanding of the signaling pathways and mechanisms involved and could ultimately provide tools for regenerative therapies for the treatment of post-MI cardiac dysfunction. We have used an in vitro human induced pluripotent stem cell-derived CPC model to screen a 10,000-compound library containing molecules representing different target classes and compounds reported to modulate the phenotype of stem or primary cells. The primary readout of this phenotypic screen was proliferation as measured by nuclear count. We identified retinoic acid receptor (RAR) agonists as potent proliferators of CPCs. The CPCs retained their progenitor phenotype following proliferation and the identified RAR agonists did not proliferate human cardiac fibroblasts, the major cell type in the heart. In addition, the RAR agonists were able to proliferate an independent source of CPCs, HuES6. The RAR agonists had a time-of-differentiation-dependent effect on the HuES6-derived CPCs. At 4 days of differentiation, treatment with retinoic acid induced differentiation of the CPCs to atrial cells. However, after 5 days of differentiation treatment with RAR agonists led to an inhibition of terminal differentiation to cardiomyocytes and enhanced the proliferation of the cells. RAR agonists, at least transiently, enhance the proliferation of human CPCs, at the expense of terminal cardiac differentiation. How this mechanism translates in vivo to activate endogenous CPCs and whether enhancing proliferation of these rare progenitor cells is sufficient to enhance cardiac repair remains to be investigated.
Keywords: cell proliferation; gene expression; high throughput screening assays; induced pluripotent stem cells; nuclear receptors; small molecule libraries.
© 2019 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
L.D. is an employee of UCB Pharma; J.M., A.N., S.P., U.K., S.M., E.M., A.D., S.K., I.B., J.S., B.M., and Q.‐D.W. are employees of AstraZeneca; A.T.P. is an employee of Sanofi; B.G. is an employee of the Max Planck Institute for Molecular Biomedicine.
Figures



Similar articles
-
Human Induced Pluripotent Stem Cell-Derived Cardiac Progenitor Cells in Phenotypic Screening: A Transforming Growth Factor-β Type 1 Receptor Kinase Inhibitor Induces Efficient Cardiac Differentiation.Stem Cells Transl Med. 2016 Feb;5(2):164-74. doi: 10.5966/sctm.2015-0114. Epub 2015 Dec 18. Stem Cells Transl Med. 2016. PMID: 26683871 Free PMC article.
-
Electrotaxis of cardiac progenitor cells, cardiac fibroblasts, and induced pluripotent stem cell-derived cardiac progenitor cells requires serum and is directed via PI3'K pathways.Heart Rhythm. 2017 Nov;14(11):1685-1692. doi: 10.1016/j.hrthm.2017.06.038. Epub 2017 Jun 28. Heart Rhythm. 2017. PMID: 28668623 Free PMC article.
-
Retinoic acid receptor signaling preserves tendon stem cell characteristics and prevents spontaneous differentiation in vitrox.Stem Cell Res Ther. 2016 Mar 22;7:45. doi: 10.1186/s13287-016-0306-3. Stem Cell Res Ther. 2016. PMID: 27001426 Free PMC article.
-
Cardiac Progenitor-Cell Derived Exosomes as Cell-Free Therapeutic for Cardiac Repair.Adv Exp Med Biol. 2017;998:207-219. doi: 10.1007/978-981-10-4397-0_14. Adv Exp Med Biol. 2017. PMID: 28936742 Review.
-
Cardiac stem cells: Current knowledge and future prospects.World J Stem Cells. 2022 Jan 26;14(1):1-40. doi: 10.4252/wjsc.v14.i1.1. World J Stem Cells. 2022. PMID: 35126826 Free PMC article. Review.
Cited by
-
Chemical Screening of Nuclear Receptor Modulators.Int J Mol Sci. 2020 Jul 31;21(15):5512. doi: 10.3390/ijms21155512. Int J Mol Sci. 2020. PMID: 32752136 Free PMC article. Review.
-
Zebrafish as a Model to Study Retinoic Acid Signaling in Development and Disease.Biomedicines. 2023 Apr 15;11(4):1180. doi: 10.3390/biomedicines11041180. Biomedicines. 2023. PMID: 37189798 Free PMC article. Review.
-
Bioactive Lipid Signaling in Cardiovascular Disease, Development, and Regeneration.Cells. 2020 Jun 3;9(6):1391. doi: 10.3390/cells9061391. Cells. 2020. PMID: 32503253 Free PMC article. Review.
-
Engineered M13 Peptide Carrier Promotes Angiogenic Potential of Patient-Derived Human Cardiac Progenitor Cells and In Vivo Engraftment.Tissue Eng Regen Med. 2020 Jun;17(3):323-333. doi: 10.1007/s13770-020-00244-w. Epub 2020 Mar 29. Tissue Eng Regen Med. 2020. PMID: 32227286 Free PMC article.
-
Phenotypic Screen with the Human Secretome Identifies FGF16 as Inducing Proliferation of iPSC-Derived Cardiac Progenitor Cells.Int J Mol Sci. 2019 Nov 30;20(23):6037. doi: 10.3390/ijms20236037. Int J Mol Sci. 2019. PMID: 31801200 Free PMC article.
References
-
- Emmert MY, Emmert LS, Martens A, et al. Higher frequencies of BCRP+ cardiac resident cells in ischaemic human myocardium. Eur Heart J. 2013;34:2830‐2838. - PubMed
-
- Guan K, Hasenfuss G. Cardiac resident progenitor cells: evidence and functional significance. Eur Heart J. 2013;34:2784‐2787. - PubMed
-
- Itzhaki‐Alfia A, Leor J, Raanani E, et al. Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation. 2009;120:2559‐2566. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

