Differentially methylated gene patterns between age-matched sarcopenic and non-sarcopenic women
- PMID: 31508907
- PMCID: PMC6903450
- DOI: 10.1002/jcsm.12478
Differentially methylated gene patterns between age-matched sarcopenic and non-sarcopenic women
Abstract
Background: Sarcopenia is characterized by progressive decreases in muscle mass, muscle strength, and muscle function with ageing. Although many studies have investigated the mechanisms of sarcopenia, its connection with epigenetic factors, such as DNA methylation, still remains poorly understood. The aim of this study was to explore sarcopenia-related DNA methylation differences in blood samples between age-matched sarcopenic and non-sarcopenic older women.
Methods: A sarcopenic group (n = 24) was identified and selected from a set of 247 older Caucasian women (aged 65-80 years) based on cut-off points of skeletal muscle index at 6.75 kg/m2 and grip strength at 26 kg (the lower quintile of grip strength in the set). A non-sarcopenic group (n = 24) was created with a similar age distribution as that of the sarcopenic group. DNA methylation patterns of whole blood samples from both groups were analysed using Infinium MethylationEPIC BeadChip arrays. Differentially methylated cytosin-phosphate-guanine sites (dmCpGs) were identified at a P value threshold of 0.01 by comparing methylation levels between the sarcopenic and non-sarcopenic groups at each CpG site. dmCpG-related genes were annotated based on Homo sapiens hg19 genome build. The functions of these genes were further examined by GO and KEGG pathway enrichment analysis.
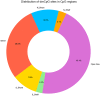
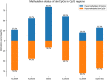
Results: The global methylation level of all analysed CpG sites (n = 788 074) showed no significant difference between the sarcopenic and non-sarcopenic groups (0.812), while the average methylation level of dmCpGs (n = 6258) was significantly lower in the sarcopenic group (0.004). The sarcopenic group had significantly higher methylation levels in TSS200 (the region from transcription start site to 200 nucleotides upstream of the site) and lower methylation levels in gene body and 3'UTR regions. In respect of CpG regions, CpG islands in promoters and some intragenic regions showed greater levels of methylation in the sarcopenic group. dmCpG-related KEGG pathways were mainly associated with muscle function, actin cytoskeleton regulation, and energy metabolism. Seven genes (HSPB1, PBX4, CNKSR3, ORMDL3, MIR10A, ZNF619, and CRADD) were found with the same methylation direction as previous studies of blood sample methylation during ageing. Fifty-four genes were shared with previous studies of resistance training.
Conclusions: Our results improve understanding of epigenetic mechanisms of sarcopenia by identifying sarcopenia-related DNA methylation differences in blood samples of older women. These methylation differences suggest underlying alterations of gene expression and pathway function, which can partially explain sarcopenia-related muscular changes.
Keywords: DNA methylation; Differentially methylated CpG sites; Older women; Pathway analysis; Sarcopenia.
© 2019 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Associations of combined genetic and epigenetic scores with muscle size and muscle strength: a pilot study in older women.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1548-1561. doi: 10.1002/jcsm.12585. Epub 2020 Oct 15. J Cachexia Sarcopenia Muscle. 2020. PMID: 33058541 Free PMC article.
-
DNA methylation of skeletal muscle function-related secretary factors identifies FGF2 as a potential biomarker for sarcopenia.J Cachexia Sarcopenia Muscle. 2024 Jun;15(3):1209-1217. doi: 10.1002/jcsm.13472. Epub 2024 Apr 20. J Cachexia Sarcopenia Muscle. 2024. PMID: 38641928 Free PMC article.
-
Epigenome-wide association study of sarcopenia: findings from the Hertfordshire Sarcopenia Study (HSS).J Cachexia Sarcopenia Muscle. 2022 Feb;13(1):240-253. doi: 10.1002/jcsm.12876. Epub 2021 Dec 4. J Cachexia Sarcopenia Muscle. 2022. PMID: 34862756 Free PMC article.
-
Genetic Associations with Aging Muscle: A Systematic Review.Cells. 2019 Dec 19;9(1):12. doi: 10.3390/cells9010012. Cells. 2019. PMID: 31861518 Free PMC article.
-
Molecular Effects of Physical Activity and Body Composition: A Systematic Review and Meta-Analysis.Int J Environ Res Public Health. 2025 Apr 18;22(4):637. doi: 10.3390/ijerph22040637. Int J Environ Res Public Health. 2025. PMID: 40283858 Free PMC article.
Cited by
-
Muscle Wasting and Sarcopenia in Heart Failure-The Current State of Science.Int J Mol Sci. 2020 Sep 8;21(18):6549. doi: 10.3390/ijms21186549. Int J Mol Sci. 2020. PMID: 32911600 Free PMC article. Review.
-
Aging and the impact of global DNA methylation, telomere shortening, and total oxidative status on sarcopenia and frailty syndrome.Immun Ageing. 2023 Nov 14;20(1):61. doi: 10.1186/s12979-023-00384-2. Immun Ageing. 2023. PMID: 37964387 Free PMC article.
-
Sex-biased expression of the TLR7 gene in severe COVID-19 patients: Insights from transcriptomics and epigenomics.Environ Res. 2022 Dec;215(Pt 2):114288. doi: 10.1016/j.envres.2022.114288. Epub 2022 Sep 21. Environ Res. 2022. PMID: 36152884 Free PMC article.
-
Associations of combined genetic and epigenetic scores with muscle size and muscle strength: a pilot study in older women.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1548-1561. doi: 10.1002/jcsm.12585. Epub 2020 Oct 15. J Cachexia Sarcopenia Muscle. 2020. PMID: 33058541 Free PMC article.
-
Epigenetics of Skeletal Muscle Atrophy.Int J Mol Sci. 2024 Jul 31;25(15):8362. doi: 10.3390/ijms25158362. Int J Mol Sci. 2024. PMID: 39125931 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

