Capillary rarefaction is more closely associated with CKD progression after cisplatin, rhabdomyolysis, and ischemia-reperfusion-induced AKI than renal fibrosis
- PMID: 31509009
- PMCID: PMC6879932
- DOI: 10.1152/ajprenal.00366.2019
Capillary rarefaction is more closely associated with CKD progression after cisplatin, rhabdomyolysis, and ischemia-reperfusion-induced AKI than renal fibrosis
Abstract
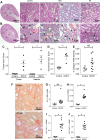
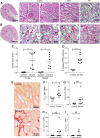
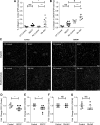
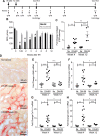
Acute kidney injury (AKI) is a strong independent predictor of mortality and often results in incomplete recovery of renal function, leading to progressive chronic kidney disease (CKD). Many clinical trials have been conducted on the basis of promising preclinical data, but no therapeutic interventions have been shown to improve long-term outcomes after AKI. This is partly due to the failure of preclinical studies to accurately model clinically relevant injury and long-term outcomes on CKD progression. Here, we evaluated the long-term effects of AKI on CKD progression in three animal models reflecting diverse etiologies of AKI: repeat-dose cisplatin, rhabdomyolysis, and ischemia-reperfusion injury. Using transdermal measurement of glomerular filtration rate as a clinically relevant measure of kidney function and quantification of peritubular capillary density to measure capillary rarefaction, we showed that repeat-dose cisplatin caused capillary rarefaction and decreased renal function in mice without a significant increase in interstitial fibrosis, whereas rhabdomyolysis-induced AKI led to severe interstitial fibrosis, but renal function and peritubular capillary density were preserved. Furthermore, long-term experiments in mice with unilateral ischemia-reperfusion injury showed that restoration of renal function 12 wk after a contralateral nephrectomy was associated with increasing fibrosis, but a reversal of capillary rarefaction was seen at 4 wk. These data demonstrate that clear dissociation between kidney function and fibrosis in these models of AKI to CKD progression and suggest that peritubular capillary rarefaction is more strongly associated with CKD progression than renal fibrosis.
Keywords: acute kidney injury; capillary rarefaction; chronic kidney disease; glomerular filtration rate; renal fibrosis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Comment in
-
The case for capillary rarefaction in the AKI to CKD progression: insights from multiple injury models.Am J Physiol Renal Physiol. 2019 Nov 1;317(5):F1253-F1254. doi: 10.1152/ajprenal.00468.2019. Epub 2019 Oct 21. Am J Physiol Renal Physiol. 2019. PMID: 31630545 No abstract available.
References
-
- Bábíčková J, Klinkhammer BM, Buhl EM, Djudjaj S, Hoss M, Heymann F, Tacke F, Floege J, Becker JU, Boor P. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int 91: 70–85, 2017. doi:10.1016/j.kint.2016.07.038. - DOI - PubMed
-
- Belliere J, Casemayou A, Ducasse L, Zakaroff-Girard A, Martins F, Iacovoni JS, Guilbeau-Frugier C, Buffin-Meyer B, Pipy B, Chauveau D, Schanstra JP, Bascands JL. Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J Am Soc Nephrol 26: 1363–1377, 2015. doi:10.1681/ASN.2014040320. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

