In utero exposure to maternal diabetes impairs nephron progenitor differentiation
- PMID: 31509011
- PMCID: PMC6879946
- DOI: 10.1152/ajprenal.00204.2019
In utero exposure to maternal diabetes impairs nephron progenitor differentiation
Abstract
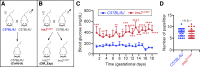
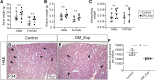
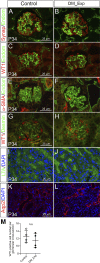
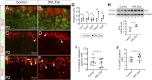
The incidence of diabetes mellitus has significantly increased among women of childbearing age, and it has been shown that prenatal exposure to maternal diabetes increases the risk of associated congenital anomalies of the kidney. Congenital anomalies of the kidney are among the leading causes of chronic kidney disease in children. To better understand the effect of maternal diabetes on kidney development, we analyzed wild-type offspring (DM_Exp) of diabetic Ins2+/C96Y mice (Akita mice). DM_Exp mice at postnatal day 34 have a reduction of ~20% in the total nephron number compared with controls, using the gold standard physical dissector/fractionator method. At the molecular level, the expression of the nephron progenitor markers sine oculis homeobox homolog 2 and Cited1 was increased in DM_Exp kidneys at postnatal day 2. Conversely, the number of early developing nephrons was diminished in DM_Exp kidneys. This was associated with decreased expression of the intracellular domain of Notch1 and the canonical Wnt target lymphoid enhancer binding factor 1. Together, these data suggest that the diabetic intrauterine environment impairs the differentiation of nephron progenitors into nephrons, possibly by perturbing the Notch and Wnt/β-catenin signaling pathways.
Keywords: diabetes; kidney development; nephron formation; nephron progenitor.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
R-spondin signalling is essential for the maintenance and differentiation of mouse nephron progenitors.Elife. 2020 May 1;9:e53895. doi: 10.7554/eLife.53895. Elife. 2020. PMID: 32324134 Free PMC article.
-
FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney.Development. 2014 Jan;141(1):17-27. doi: 10.1242/dev.089078. Epub 2013 Nov 27. Development. 2014. PMID: 24284212 Free PMC article.
-
Prorenin receptor is critical for nephron progenitors.Dev Biol. 2016 Jan 15;409(2):382-91. doi: 10.1016/j.ydbio.2015.11.024. Epub 2015 Dec 3. Dev Biol. 2016. PMID: 26658320 Free PMC article.
-
Epigenetic States of nephron progenitors and epithelial differentiation.J Cell Biochem. 2015 Jun;116(6):893-902. doi: 10.1002/jcb.25048. J Cell Biochem. 2015. PMID: 25560433 Free PMC article. Review.
-
Kidney Nephron Determination.Annu Rev Cell Dev Biol. 2018 Oct 6;34:427-450. doi: 10.1146/annurev-cellbio-100616-060647. Epub 2018 Aug 20. Annu Rev Cell Dev Biol. 2018. PMID: 30125139 Review.
Cited by
-
In utero exposure to maternal diabetes exacerbates dietary sodium intake-induced endothelial dysfunction by activating cyclooxygenase 2-derived prostanoids.Am J Physiol Endocrinol Metab. 2024 May 1;326(5):E555-E566. doi: 10.1152/ajpendo.00009.2024. Epub 2024 Mar 6. Am J Physiol Endocrinol Metab. 2024. PMID: 38446637 Free PMC article.
-
Notch signaling in diabetic kidney disease: recent progress.Front Endocrinol (Lausanne). 2025 Jul 31;16:1537769. doi: 10.3389/fendo.2025.1537769. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40822939 Free PMC article. Review.
-
Perinatal risk factors associated with acute kidney injury severity and duration among infants born extremely preterm.Pediatr Res. 2024 Aug;96(3):740-749. doi: 10.1038/s41390-024-03102-w. Epub 2024 Mar 4. Pediatr Res. 2024. PMID: 38438550 Free PMC article.
-
The Impact of Gestational Diabetes on Kidney Development: is There an Epigenetic Link?Curr Diab Rep. 2024 Dec 18;25(1):13. doi: 10.1007/s11892-024-01569-9. Curr Diab Rep. 2024. PMID: 39690358 Free PMC article. Review.
-
How Stem and Progenitor Cells Can Affect Renal Diseases.Cells. 2024 Aug 30;13(17):1460. doi: 10.3390/cells13171460. Cells. 2024. PMID: 39273032 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

