Individualized management of genetic diversity in Niemann-Pick C1 through modulation of the Hsp70 chaperone system
- PMID: 31509197
- PMCID: PMC7001602
- DOI: 10.1093/hmg/ddz215
Individualized management of genetic diversity in Niemann-Pick C1 through modulation of the Hsp70 chaperone system
Abstract
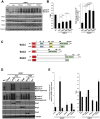
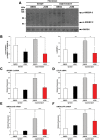
Genetic diversity provides a rich repository for understanding the role of proteostasis in the management of the protein fold in human biology. Failure in proteostasis can trigger multiple disease states, affecting both human health and lifespan. Niemann-Pick C1 (NPC1) disease is a rare genetic disorder triggered by mutations in NPC1, a multi-spanning transmembrane protein that is trafficked through the exocytic pathway to late endosomes (LE) and lysosomes (Ly) (LE/Ly) to globally manage cholesterol homeostasis. Defects triggered by >300 NPC1 variants found in the human population inhibit export of NPC1 protein from the endoplasmic reticulum (ER) and/or function in downstream LE/Ly, leading to cholesterol accumulation and onset of neurodegeneration in childhood. We now show that the allosteric inhibitor JG98, that targets the cytosolic Hsp70 chaperone/co-chaperone complex, can significantly improve the trafficking and post-ER protein level of diverse NPC1 variants. Using a new approach to model genetic diversity in human disease, referred to as variation spatial profiling, we show quantitatively how JG98 alters the Hsp70 chaperone/co-chaperone system to adjust the spatial covariance (SCV) tolerance and set-points on an amino acid residue-by-residue basis in NPC1 to differentially regulate variant trafficking, stability, and cholesterol homeostasis, results consistent with the role of BCL2-associated athanogene family co-chaperones in managing the folding status of NPC1 variants. We propose that targeting the cytosolic Hsp70 system by allosteric regulation of its chaperone/co-chaperone based client relationships can be used to adjust the SCV tolerance of proteostasis buffering capacity to provide an approach to mitigate systemic and neurological disease in the NPC1 population.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Quantitating the epigenetic transformation contributing to cholesterol homeostasis using Gaussian process.Nat Commun. 2019 Nov 7;10(1):5052. doi: 10.1038/s41467-019-12969-x. Nat Commun. 2019. PMID: 31699992 Free PMC article.
-
HSP90 inhibitors reduce cholesterol storage in Niemann-Pick type C1 mutant fibroblasts.J Lipid Res. 2021;62:100114. doi: 10.1016/j.jlr.2021.100114. Epub 2021 Sep 3. J Lipid Res. 2021. PMID: 34481829 Free PMC article.
-
Beneficial effects of primidone in Niemann-Pick disease type C (NPC)-model cells and mice: Reduction of unesterified cholesterol levels in cells and extension of lifespan in mice.Eur J Pharmacol. 2021 Apr 5;896:173907. doi: 10.1016/j.ejphar.2021.173907. Epub 2021 Jan 24. Eur J Pharmacol. 2021. PMID: 33503462
-
Lysosome and endoplasmic reticulum quality control pathways in Niemann-Pick type C disease.Brain Res. 2016 Oct 15;1649(Pt B):181-188. doi: 10.1016/j.brainres.2016.03.035. Epub 2016 Mar 26. Brain Res. 2016. PMID: 27026653 Free PMC article. Review.
-
Treatment trials in Niemann-Pick type C disease.Metab Brain Dis. 2021 Dec;36(8):2215-2221. doi: 10.1007/s11011-021-00842-0. Epub 2021 Oct 1. Metab Brain Dis. 2021. PMID: 34596813 Free PMC article. Review.
Cited by
-
Proteostasis Regulators Restore Function of Epilepsy-Associated GABAA Receptors.Cell Chem Biol. 2021 Jan 21;28(1):46-59.e7. doi: 10.1016/j.chembiol.2020.08.012. Epub 2020 Sep 3. Cell Chem Biol. 2021. PMID: 32888501 Free PMC article.
-
Quantitating the epigenetic transformation contributing to cholesterol homeostasis using Gaussian process.Nat Commun. 2019 Nov 7;10(1):5052. doi: 10.1038/s41467-019-12969-x. Nat Commun. 2019. PMID: 31699992 Free PMC article.
-
Alterations in Proteostasis Mechanisms in Niemann-Pick Type C Disease.Int J Mol Sci. 2024 Mar 29;25(7):3806. doi: 10.3390/ijms25073806. Int J Mol Sci. 2024. PMID: 38612616 Free PMC article. Review.
-
Image-based screen capturing misfolding status of Niemann-Pick type C1 identifies potential candidates for chaperone drugs.PLoS One. 2020 Dec 14;15(12):e0243746. doi: 10.1371/journal.pone.0243746. eCollection 2020. PLoS One. 2020. PMID: 33315900 Free PMC article.
-
Exploration of Bromodomain Proteins as Drug Targets for Niemann-Pick Type C Disease.Int J Mol Sci. 2025 Jun 16;26(12):5769. doi: 10.3390/ijms26125769. Int J Mol Sci. 2025. PMID: 40565243 Free PMC article.
References
-
- Torkamani A., Wineinger N.E. and Topol E.J. (2018) The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet., 19, 581–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

