Forkhead transcription factor FOXO3a mediates interferon-γ-induced MHC II transcription in macrophages
- PMID: 31509237
- PMCID: PMC6856938
- DOI: 10.1111/imm.13116
Forkhead transcription factor FOXO3a mediates interferon-γ-induced MHC II transcription in macrophages
Abstract
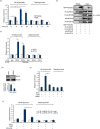
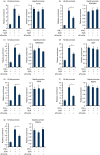
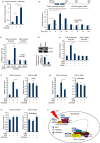
Macrophages are professional antigen-presenting cells relying on the expression of class II major histocompatibility complex (MHC II) genes. Interferon-γ (IFN-γ) activates MHC II transcription via the assembly of an enhanceosome centred on class II trans-activator (CIITA). In the present study, we investigated the role of the forkhead transcription factor FOXO3a in IFN- γ-induced MHC II transcription in macrophages. Knockdown of FOXO3a, but not FOXO1 or FOXO4, diminished IFN-γ-induced MHC II expression in RAW cells. On the contrary, over-expression of FOXO3a, but neither FOXO1 nor FOXO4, enhanced CIITA-mediated trans-activation of the MHC II promoter. IFN-γ treatment promoted the recruitment of FOXO3a to the MHC II promoter. Co-immunoprecipitation and RE-ChIP assays showed that FOXO3a was a component of the MHC II enhanceosome forming interactions with CIITA, RFX5, RFXB and RFXAP. FOXO3a contributed to MHC II transcription by altering histone modifications surrounding the MHC II promoter. Of interest, FOXO3a was recruited to the type IV CIITA promoter and directly activated CIITA transcription by interacting with signal transducer of activation and transcription 1 in response to IFN-γ stimulation. In conclusion, our data unveil a novel role for FOXO3a in the regulation of MHC II transcription in macrophages.
Keywords: macrophage; FOXO; epigenetics; transcriptional regulation.
© 2019 John Wiley & Sons Ltd.
Figures

Similar articles
-
Histone deacetylase 1/mSin3A disrupts gamma interferon-induced CIITA function and major histocompatibility complex class II enhanceosome formation.Mol Cell Biol. 2003 May;23(9):3091-102. doi: 10.1128/MCB.23.9.3091-3102.2003. Mol Cell Biol. 2003. PMID: 12697811 Free PMC article.
-
IFN-gamma regulation of class II transactivator promoter IV in macrophages and microglia: involvement of the suppressors of cytokine signaling-1 protein.J Immunol. 2001 Feb 15;166(4):2260-9. doi: 10.4049/jimmunol.166.4.2260. J Immunol. 2001. PMID: 11160280
-
Collagen and major histocompatibility class II expression in mesenchymal cells from CIITA hypomorphic mice.Mol Immunol. 2007 Mar;44(7):1709-21. doi: 10.1016/j.molimm.2006.07.294. Epub 2006 Sep 18. Mol Immunol. 2007. PMID: 16982097
-
Expression of MHC II genes.Curr Top Microbiol Immunol. 2005;290:147-70. doi: 10.1007/3-540-26363-2_7. Curr Top Microbiol Immunol. 2005. PMID: 16480042 Review.
-
Structural aspects of the MHC expression control system.Biophys Chem. 2022 May;284:106781. doi: 10.1016/j.bpc.2022.106781. Epub 2022 Feb 15. Biophys Chem. 2022. PMID: 35228036 Free PMC article. Review.
Cited by
-
Novel Immune-Related Gene Signature for Risk Stratification and Prognosis of Survival in Lower-Grade Glioma.Front Genet. 2020 Apr 15;11:363. doi: 10.3389/fgene.2020.00363. eCollection 2020. Front Genet. 2020. PMID: 32351547 Free PMC article.
-
Immunological Involvement of MicroRNAs in the Key Events of Systemic Lupus Erythematosus.Front Immunol. 2021 Aug 2;12:699684. doi: 10.3389/fimmu.2021.699684. eCollection 2021. Front Immunol. 2021. PMID: 34408748 Free PMC article. Review.
-
Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19.Open Biol. 2021 Jun;11(6):210069. doi: 10.1098/rsob.210069. Epub 2021 Jun 9. Open Biol. 2021. PMID: 34102081 Free PMC article. Review.
-
Epigenetic targeting of the ACE2 and NRP1 viral receptors limits SARS-CoV-2 infectivity.Clin Epigenetics. 2021 Oct 11;13(1):187. doi: 10.1186/s13148-021-01168-5. Clin Epigenetics. 2021. PMID: 34635175 Free PMC article.
-
Prognostic value of four immune-related genes in lower-grade gliomas: a biomarker discovery study.Front Genet. 2024 Aug 12;15:1403587. doi: 10.3389/fgene.2024.1403587. eCollection 2024. Front Genet. 2024. PMID: 39192888 Free PMC article.
References
-
- Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol 2001; 19:331–73. - PubMed
-
- Masternak K, Muhlethaler‐Mottet A, Villard J, Peretti M, Reith W. Molecular genetics of the bare lymphocyte syndrome. Rev Immunogenet 2000; 2:267–82. - PubMed
-
- Friese MA, Jones EY, Fugger L. MHC II molecules in inflammatory diseases: interplay of qualities and quantities. Trends Immunol 2005; 26:559–61. - PubMed
-
- Reith W, Muhlethaler‐Mottet A, Masternak K, Villard J, Mach B. The molecular basis of MHC class II deficiency and transcriptional control of MHC class II gene expression. Microbes Infect 1999; 1:839–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

