Intestinal epithelial cells: at the interface of the microbiota and mucosal immunity
- PMID: 31509239
- PMCID: PMC6856932
- DOI: 10.1111/imm.13117
Intestinal epithelial cells: at the interface of the microbiota and mucosal immunity
Abstract
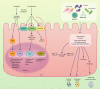
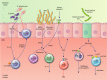
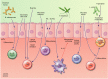
The intestinal epithelium forms a barrier between the microbiota and the rest of the body. In addition, beyond acting as a physical barrier, the function of intestinal epithelial cells (IECs) in sensing and responding to microbial signals is increasingly appreciated and likely has numerous implications for the vast network of immune cells within and below the intestinal epithelium. IECs also respond to factors produced by immune cells, and these can regulate IEC barrier function, proliferation and differentiation, as well as influence the composition of the microbiota. The mechanisms involved in IEC-microbe-immune interactions, however, are not fully characterized. In this review, we explore the ability of IECs to direct intestinal homeostasis by orchestrating communication between intestinal microbes and mucosal innate and adaptive immune cells during physiological and inflammatory conditions. We focus primarily on the most recent findings and call attention to the numerous remaining unknowns regarding the complex crosstalk between IECs, the microbiota and intestinal immune cells.
Keywords: bacterial; epithelial cell; gut; microbiota; mucosal immunology.
© 2019 John Wiley & Sons Ltd.
Figures

Similar articles
-
The Intestinal Epithelium: Central Coordinator of Mucosal Immunity.Trends Immunol. 2018 Sep;39(9):677-696. doi: 10.1016/j.it.2018.04.002. Epub 2018 Apr 30. Trends Immunol. 2018. PMID: 29716793 Review.
-
Epithelial-immune cell crosstalk for intestinal barrier homeostasis.Eur J Immunol. 2024 Jun;54(6):e2350631. doi: 10.1002/eji.202350631. Epub 2024 Mar 31. Eur J Immunol. 2024. PMID: 38556632 Review.
-
Epithelial crosstalk at the microbiota-mucosal interface.Proc Natl Acad Sci U S A. 2011 Mar 15;108 Suppl 1(Suppl 1):4607-14. doi: 10.1073/pnas.1000092107. Epub 2010 Sep 8. Proc Natl Acad Sci U S A. 2011. PMID: 20826446 Free PMC article. Review.
-
The microbiome and regulation of mucosal immunity.Immunology. 2014 May;142(1):24-31. doi: 10.1111/imm.12231. Immunology. 2014. PMID: 24329495 Free PMC article. Review.
-
Epithelial immunity: priming defensive responses in the intestinal mucosa.Am J Physiol Gastrointest Liver Physiol. 2018 Feb 1;314(2):G247-G255. doi: 10.1152/ajpgi.00215.2016. Epub 2017 Nov 16. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29146676 Review.
Cited by
-
Clinical predictive value of the initial neutrophils to lymphocytes and platelets ratio for prognosis of sepsis patients in the intensive care unit: a retrospective study.Front Med (Lausanne). 2024 Jan 18;11:1351492. doi: 10.3389/fmed.2024.1351492. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38318247 Free PMC article.
-
Sirtuin 6 maintains epithelial STAT6 activity to support intestinal tuft cell development and type 2 immunity.Nat Commun. 2022 Sep 3;13(1):5192. doi: 10.1038/s41467-022-32846-4. Nat Commun. 2022. PMID: 36057627 Free PMC article.
-
Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases.Nutrients. 2021 Mar 5;13(3):850. doi: 10.3390/nu13030850. Nutrients. 2021. PMID: 33807621 Free PMC article. Review.
-
The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut?Int J Mol Sci. 2022 Jan 27;23(3):1494. doi: 10.3390/ijms23031494. Int J Mol Sci. 2022. PMID: 35163417 Free PMC article. Review.
-
Bacterial and Viral Co-Infection in the Intestine: Competition Scenario and Their Effect on Host Immunity.Int J Mol Sci. 2022 Feb 19;23(4):2311. doi: 10.3390/ijms23042311. Int J Mol Sci. 2022. PMID: 35216425 Free PMC article. Review.
References
-
- Parikh K, Antanaviciute A, Fawkner‐Corbett D, Jagielowicz M, Aulicino A, Lagerholm C et al Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019; 567:49–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

