Photoinduced Electron Transfer in a Radical SAM Enzyme Generates an S-Adenosylmethionine Derived Methyl Radical
- PMID: 31509404
- PMCID: PMC6901192
- DOI: 10.1021/jacs.9b08541
Photoinduced Electron Transfer in a Radical SAM Enzyme Generates an S-Adenosylmethionine Derived Methyl Radical
Abstract
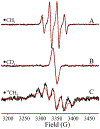
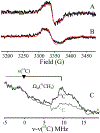
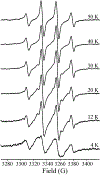
Radical SAM (RS) enzymes use S-adenosyl-l-methionine (SAM) and a [4Fe-4S] cluster to initiate a broad spectrum of radical transformations throughout all kingdoms of life. We report here that low-temperature photoinduced electron transfer from the [4Fe-4S]1+ cluster to bound SAM in the active site of the hydrogenase maturase RS enzyme, HydG, results in specific homolytic cleavage of the S-CH3 bond of SAM, rather than the S-C5' bond as in the enzyme-catalyzed (thermal) HydG reaction. This result is in stark contrast to a recent report in which photoinduced ET in the RS enzyme pyruvate formate-lyase activating enzyme cleaved the S-C5' bond to generate a 5'-deoxyadenosyl radical, and provides the first direct evidence for homolytic S-CH3 bond cleavage in a RS enzyme. Photoinduced ET in HydG generates a trapped •CH3 radical, as well as a small population of an organometallic species with an Fe-CH3 bond, denoted ΩM. The •CH3 radical is surprisingly found to exhibit rotational diffusion in the HydG active site at temperatures as low as 40 K, and is rapidly quenched: whereas 5'-dAdo• is stable indefinitely at 77 K, •CH3 quenches with a half-time of ∼2 min at this temperature. The rapid quenching and rotational/translational freedom of •CH3 shows that enzymes would be unable to harness this radical as a regio- and stereospecific H atom abstractor during catalysis, in contrast to the exquisite control achieved with the enzymatically generated 5'-dAdo•.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

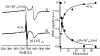
Similar articles
-
Active-Site Controlled, Jahn-Teller Enabled Regioselectivity in Reductive S-C Bond Cleavage of S-Adenosylmethionine in Radical SAM Enzymes.J Am Chem Soc. 2021 Jan 13;143(1):335-348. doi: 10.1021/jacs.0c10925. Epub 2020 Dec 29. J Am Chem Soc. 2021. PMID: 33372786 Free PMC article.
-
ENDOR Spectroscopy Reveals the "Free" 5'-Deoxyadenosyl Radical in a Radical SAM Enzyme Active Site Actually is Chaperoned by Close Interaction with the Methionine-Bound [4Fe-4S]2+ Cluster.J Am Chem Soc. 2024 Feb 14;146(6):3710-3720. doi: 10.1021/jacs.3c09428. Epub 2024 Feb 3. J Am Chem Soc. 2024. PMID: 38308759 Free PMC article.
-
Radical SAM Enzyme Spore Photoproduct Lyase: Properties of the Ω Organometallic Intermediate and Identification of Stable Protein Radicals Formed during Substrate-Free Turnover.J Am Chem Soc. 2020 Oct 28;142(43):18652-18660. doi: 10.1021/jacs.0c08585. Epub 2020 Oct 15. J Am Chem Soc. 2020. PMID: 32966073 Free PMC article.
-
Mechanism of Radical Initiation in the Radical S-Adenosyl-l-methionine Superfamily.Acc Chem Res. 2018 Nov 20;51(11):2611-2619. doi: 10.1021/acs.accounts.8b00356. Epub 2018 Oct 15. Acc Chem Res. 2018. PMID: 30346729 Free PMC article. Review.
-
Radical SAM enzymes: Nature's choice for radical reactions.FEBS Lett. 2023 Jan;597(1):92-101. doi: 10.1002/1873-3468.14519. Epub 2022 Oct 27. FEBS Lett. 2023. PMID: 36251330 Free PMC article. Review.
Cited by
-
Characterization by ENDOR Spectroscopy of the Iron-Alkyl Bond in a Synthetic Counterpart of Organometallic Intermediates in Radical SAM Enzymes.J Am Chem Soc. 2022 Sep 28;144(38):17642-17650. doi: 10.1021/jacs.2c07155. Epub 2022 Sep 15. J Am Chem Soc. 2022. PMID: 36108299 Free PMC article.
-
Mechanism of Radical S-Adenosyl-l-methionine Adenosylation: Radical Intermediates and the Catalytic Competence of the 5'-Deoxyadenosyl Radical.J Am Chem Soc. 2022 Mar 23;144(11):5087-5098. doi: 10.1021/jacs.1c13706. Epub 2022 Mar 8. J Am Chem Soc. 2022. PMID: 35258967 Free PMC article.
-
Biosynthesis of the [FeFe] hydrogenase H-cluster via a synthetic [Fe(II)(CN)(CO)2(cysteinate)]- complex.Dalton Trans. 2021 Sep 21;50(36):12386-12391. doi: 10.1039/d1dt02258j. Dalton Trans. 2021. PMID: 34545884 Free PMC article. Review.
-
HydG, the "dangler" iron, and catalytic production of free CO and CN-: implications for [FeFe]-hydrogenase maturation.Dalton Trans. 2021 Aug 4;50(30):10405-10422. doi: 10.1039/d1dt01359a. Dalton Trans. 2021. PMID: 34240096 Free PMC article.
-
Manipulating D-A interaction to achieve stable photoinduced organic radicals in triphenylphosphine crystals.Chem Sci. 2023 Jan 18;14(7):1871-1877. doi: 10.1039/d2sc05753k. eCollection 2023 Feb 15. Chem Sci. 2023. PMID: 36819874 Free PMC article.
References
-
- Banerjee R; Ragsdale SW The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem 2003, 72, 209–247. - PubMed
-
- Brown KL Chemistry and Enzymology of Vitamin B12. Chem. Rev 2005, 105, 2075–2149. - PubMed
-
- Toraya T Radical catalysis in coenzyme B-12-dependent isomerization (eliminating) reactions. Chem. Rev 2003, 103, 2095–2127. - PubMed
-
- Frey PA Lysine 2,3-aminomutase: is adenosylmethionine a poor man’s adenosylcobalamin? FASEB J. 1993, 7, 662–670. - PubMed
-
- Frey PA; Magnusson OT S-Adenosylmethionine: A wolf in sheep’s clothing, or a rich man’s adenosylcobalamin? Chem. Rev 2003, 103, 2129–2148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

