Clinically relevant model of pneumococcal pneumonia, ARDS, and nonpulmonary organ dysfunction in mice
- PMID: 31509438
- PMCID: PMC6879898
- DOI: 10.1152/ajplung.00132.2019
Clinically relevant model of pneumococcal pneumonia, ARDS, and nonpulmonary organ dysfunction in mice
Abstract
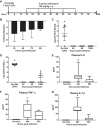
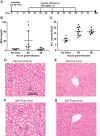
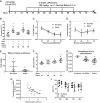
Pneumonia is responsible for more deaths in the United States than any other infectious disease. Severe pneumonia is a common cause of acute respiratory failure and acute respiratory distress syndrome (ARDS). Despite the introduction of effective antibiotics and intensive supportive care in the 20th century, death rates from community-acquired pneumonia among patients in the intensive care unit remain as high as 35%. Beyond antimicrobial treatment, no targeted molecular therapies have yet proven effective, highlighting the need for additional research. Despite some limitations, small animal models of pneumonia and the mechanistic insights they produce are likely to continue to play an important role in generating new therapeutic targets. Here we describe the development of an innovative mouse model of pneumococcal pneumonia developed for enhanced clinical relevance. We first reviewed the literature of small animal models of bacterial pneumonia that incorporated antibiotics. We then did a series of experiments in mice in which we systematically varied the pneumococcal inoculum and the timing of antibiotics while measuring systemic and lung-specific end points, producing a range of models that mirrors the spectrum of pneumococcal lung disease in patients, from mild self-resolving infection to severe pneumonia refractory to antibiotics. A delay in antibiotic treatment resulted in ongoing inflammation and renal and hepatic dysfunction despite effective bacterial killing. The addition of fluid resuscitation to the model improved renal function but worsened the severity of lung injury based on direct measurements of pulmonary edema and lung compliance, analogous to patients with pneumonia and sepsis who develop ARDS following fluid administration.
Keywords: ARDS; acute lung injury; pneumococcus; pneumonia; sepsis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
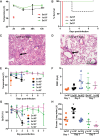
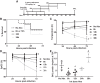
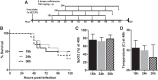

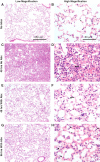

Similar articles
-
Adult respiratory distress syndrome (ARDS) due to bacteraemic pneumococcal pneumonia.Eur Respir J. 1991 Apr;4(4):503-4. Eur Respir J. 1991. PMID: 1855580
-
Cigarette smoke exposure worsens acute lung injury in antibiotic-treated bacterial pneumonia in mice.Am J Physiol Lung Cell Mol Physiol. 2018 Jul 1;315(1):L25-L40. doi: 10.1152/ajplung.00405.2017. Epub 2018 Mar 15. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29543040 Free PMC article.
-
Adult respiratory distress syndrome as a cause of death in pneumococcal pneumonia. Report of ten cases.Chest. 1983 Apr;83(4):598-601. doi: 10.1378/chest.83.4.598. Chest. 1983. PMID: 6831945
-
Sepsis syndrome, the adult respiratory distress syndrome, and nosocomial pneumonia. A common clinical sequence.Clin Chest Med. 1990 Dec;11(4):633-56. Clin Chest Med. 1990. PMID: 2268994 Review.
-
Acute respiratory distress syndrome and acute lung injury.Postgrad Med J. 2011 Sep;87(1031):612-22. doi: 10.1136/pgmj.2011.118398. Epub 2011 Jun 4. Postgrad Med J. 2011. PMID: 21642654 Review.
Cited by
-
The endogenous capacity to produce proinflammatory mediators by the ex vivo human perfused lung.Intensive Care Med Exp. 2020 Sep 21;8(1):56. doi: 10.1186/s40635-020-00343-x. Intensive Care Med Exp. 2020. PMID: 32955627 Free PMC article.
-
Role of Cholesterol 25-Hydroxylase (Ch25h) in Mediating Innate Immune Responses to Streptococcus pneumoniae Infection.Cells. 2023 Feb 10;12(4):570. doi: 10.3390/cells12040570. Cells. 2023. PMID: 36831236 Free PMC article.
-
Biological Effects of Corticosteroids on Pneumococcal Pneumonia in Mice and Humans.Res Sq [Preprint]. 2024 Feb 21:rs.3.rs-3962861. doi: 10.21203/rs.3.rs-3962861/v1. Res Sq. 2024. Update in: Crit Care. 2024 May 29;28(1):185. doi: 10.1186/s13054-024-04956-6. PMID: 38464245 Free PMC article. Updated. Preprint.
-
Understanding the role of neutrophils in acute respiratory distress syndrome.Biomed J. 2021 Aug;44(4):439-446. doi: 10.1016/j.bj.2020.09.001. Epub 2020 Sep 10. Biomed J. 2021. PMID: 33087299 Free PMC article. Review.
-
The future of sepsis research: time to think differently?Am J Physiol Lung Cell Mol Physiol. 2020 Sep 1;319(3):L523-L526. doi: 10.1152/ajplung.00368.2020. Epub 2020 Aug 5. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32755382 Free PMC article. No abstract available.
References
-
- Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, Ai X, Serhan CN, Levy BD. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol 9: 1278–1287, 2016. doi:10.1038/mi.2015.129. - DOI - PMC - PubMed
-
- Bast DJ, Yue M, Chen X, Bell D, Dresser L, Saskin R, Mandell LA, Low DE, de Azavedo JC. Novel murine model of pneumococcal pneumonia: use of temperature as a measure of disease severity to compare the efficacies of moxifloxacin and levofloxacin. Antimicrob Agents Chemother 48: 3343–3348, 2004. doi:10.1128/AAC.48.9.3343-3348.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

