Bnip3 regulates airway smooth muscle cell focal adhesion and proliferation
- PMID: 31509440
- PMCID: PMC6957365
- DOI: 10.1152/ajplung.00224.2019
Bnip3 regulates airway smooth muscle cell focal adhesion and proliferation
Abstract
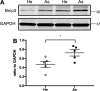
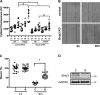
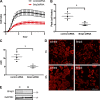
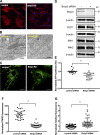
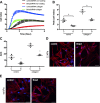
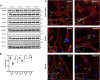
Increased airway smooth muscle (ASM) mass is a key contributor to airway narrowing and airway hyperresponsiveness in asthma. Besides conventional pathways and regulators of ASM proliferation, recent studies suggest that changes in mitochondrial morphology and function play a role in airway remodeling in asthma. In this study, we aimed at determining the role of mitochondrial Bcl-2 adenovirus E1B 19 kDa-interacting protein, Bnip3, in the regulation of ASM proliferation. Bnip3 is a member of the Bcl-2 family of proteins critical for mitochondrial health, mitophagy, and cell survival/death. We found that Bnip3 expression is upregulated in ASM cells from asthmatic donors compared with that in ASM cells from healthy donors and transient downregulation of Bnip3 expression in primary human ASM cells using an siRNA approach decreased cell adhesion, migration, and proliferation. Furthermore, Bnip3 downregulation altered the structure (electron density) and function (cellular ATP levels, membrane potential, and reacitve oxygen species generation) of mitochondria and decreased expression of cytoskeleton proteins vinculin, paxillin, and actinin. These findings suggest that Bnip3 via regulation of mitochondria functions and expression of adhesion proteins regulates ASM adhesion, migration, and proliferation. This study reveals a novel role for Bnip3 in ASM functions and establishes Bnip3 as a potential target in mitigating ASM remodeling in asthma.
Keywords: Bnip3; asthma; growth; remodeling.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Comment in
-
Bnip3 as a potential target to treat airway smooth muscle remodeling in asthma?Am J Physiol Lung Cell Mol Physiol. 2020 Jan 1;318(1):L212. doi: 10.1152/ajplung.00431.2019. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31910033 No abstract available.
-
Reply to Letter to the Editor: "Bnip3 as a potential target to treat airway smooth muscle remodeling in asthma?".Am J Physiol Lung Cell Mol Physiol. 2020 Jan 1;318(1):L213-L214. doi: 10.1152/ajplung.00470.2019. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31910034 No abstract available.
Similar articles
-
Bitter taste receptor agonists alter mitochondrial function and induce autophagy in airway smooth muscle cells.Am J Physiol Lung Cell Mol Physiol. 2017 Jul 1;313(1):L154-L165. doi: 10.1152/ajplung.00106.2017. Epub 2017 Apr 27. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28450286 Free PMC article.
-
MicroRNA-221 is overexpressed in the equine asthmatic airway smooth muscle and modulates smooth muscle cell proliferation.Am J Physiol Lung Cell Mol Physiol. 2019 Dec 1;317(6):L748-L757. doi: 10.1152/ajplung.00221.2018. Epub 2019 Aug 7. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31389734
-
Neuronal chemorepellent Semaphorin 3E inhibits human airway smooth muscle cell proliferation and migration.J Allergy Clin Immunol. 2014 Feb;133(2):560-7. doi: 10.1016/j.jaci.2013.06.011. Epub 2013 Aug 6. J Allergy Clin Immunol. 2014. PMID: 23932461
-
Bnip3 in mitophagy: Novel insights and potential therapeutic target for diseases of secondary mitochondrial dysfunction.Clin Chim Acta. 2020 Jul;506:72-83. doi: 10.1016/j.cca.2020.02.024. Epub 2020 Feb 21. Clin Chim Acta. 2020. PMID: 32092316 Review.
-
Airway remodeling in asthma: new mechanisms and potential for pharmacological intervention.Pharmacol Ther. 2011 Jun;130(3):325-37. doi: 10.1016/j.pharmthera.2011.02.001. Epub 2011 Feb 17. Pharmacol Ther. 2011. PMID: 21334378 Review.
Cited by
-
Targeting Airway Smooth Muscle Hypertrophy in Asthma: An Approach Whose Time Has Come.J Asthma Allergy. 2021 May 25;14:539-556. doi: 10.2147/JAA.S280247. eCollection 2021. J Asthma Allergy. 2021. PMID: 34079293 Free PMC article. Review.
-
Mitochondrial dysfunction in lung ageing and disease.Eur Respir Rev. 2020 Oct 15;29(157):200165. doi: 10.1183/16000617.0165-2020. Print 2020 Sep 30. Eur Respir Rev. 2020. PMID: 33060165 Free PMC article. Review.
-
Mechanisms of Immune-Related Long Non-Coding RNAs in Spleens of Mice Vaccinated with 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23).Vaccines (Basel). 2023 Feb 23;11(3):529. doi: 10.3390/vaccines11030529. Vaccines (Basel). 2023. PMID: 36992112 Free PMC article.
-
Oxidative Stress Mediates the Dual Regulatory Effects of Bovine Uterine ECM Remodeling Through the TGF-β1/Smad3 Pathway: Molecular Mechanisms of MMPs and COL-IV Imbalances.Animals (Basel). 2025 Jun 23;15(13):1847. doi: 10.3390/ani15131847. Animals (Basel). 2025. PMID: 40646747 Free PMC article.
-
Mitochondrial Dysfunction in Chronic Respiratory Diseases: Implications for the Pathogenesis and Potential Therapeutics.Oxid Med Cell Longev. 2021 Jul 27;2021:5188306. doi: 10.1155/2021/5188306. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34354793 Free PMC article. Review.
References
-
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345, 2011. doi:10.1038/nature10234. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

