Responses of neurons in macaque MT to unikinetic plaids
- PMID: 31509468
- PMCID: PMC6879962
- DOI: 10.1152/jn.00486.2019
Responses of neurons in macaque MT to unikinetic plaids
Abstract
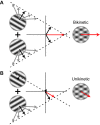
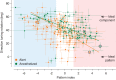
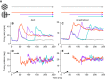
Response properties of MT neurons are often studied with "bikinetic" plaid stimuli, which consist of two superimposed sine wave gratings moving in different directions. Oculomotor studies using "unikinetic plaids" in which only one of the two superimposed gratings moves suggest that the eyes first move reflexively in the direction of the moving grating and only later converge on the perceived direction of the moving pattern. MT has been implicated as the source of visual signals that drives these responses. We wanted to know whether stationary gratings, which have little effect on MT cells when presented alone, would influence MT responses when paired with a moving grating. We recorded extracellularly from neurons in area MT and measured responses to stationary and moving gratings, and to their sums: bikinetic and unikinetic plaids. As expected, stationary gratings presented alone had a very modest influence on the activity of MT neurons. Responses to moving gratings and bikinetic plaids were similar to those previously reported and revealed cells selective for the motion of plaid patterns and of their components (pattern and component cells). When these neurons were probed with unikinetic plaids, pattern cells shifted their direction preferences in a way that revealed the influence of the static grating. Component cell preferences shifted little or not at all. These results support the notion that pattern-selective neurons in area MT integrate component motions that differ widely in speed, and that they do so in a way that is consistent with an intersection-of-constraints model.NEW & NOTEWORTHY Human perceptual and eye movement responses to moving gratings are influenced by adding a second, static grating to create a "unikinetic" plaid. Cells in MT do not respond to static gratings, but those gratings still influence the direction selectivity of some MT cells. The cells influenced by static gratings are those tuned for the motion of global patterns, but not those tuned only for the individual components of moving targets.
Keywords: MT; direction selectivity; electrophysiology; motion integration; motion perception.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Dynamics of macaque MT cell responses to grating triplets.J Neurosci. 2012 Jun 13;32(24):8242-53. doi: 10.1523/JNEUROSCI.5787-11.2012. J Neurosci. 2012. PMID: 22699905 Free PMC article.
-
Perceptual, oculomotor, and neural responses to moving color plaids.Perception. 1998;27(6):681-709. doi: 10.1068/p270681. Perception. 1998. PMID: 10197187
-
Binocular integration of pattern motion signals by MT neurons and by human observers.J Neurosci. 2010 May 26;30(21):7344-9. doi: 10.1523/JNEUROSCI.4552-09.2010. J Neurosci. 2010. PMID: 20505101 Free PMC article.
-
Temporal and spatial limits of pattern motion sensitivity in macaque MT neurons.J Neurophysiol. 2015 Apr 1;113(7):1977-88. doi: 10.1152/jn.00597.2014. Epub 2014 Dec 24. J Neurophysiol. 2015. PMID: 25540222 Free PMC article.
-
Computing motion in the primate's visual system.J Exp Biol. 1989 Sep;146:115-39. doi: 10.1242/jeb.146.1.115. J Exp Biol. 1989. PMID: 2689558 Review.
Cited by
-
But Still It Moves: Static Image Statistics Underlie How We See Motion.J Neurosci. 2020 Mar 18;40(12):2538-2552. doi: 10.1523/JNEUROSCI.2760-19.2020. Epub 2020 Feb 13. J Neurosci. 2020. PMID: 32054676 Free PMC article.
-
Shared Mechanisms Drive Ocular Following and Motion Perception.eNeuro. 2024 Jun 20;11(6):ENEURO.0204-24.2024. doi: 10.1523/ENEURO.0204-24.2024. Print 2024 Jun. eNeuro. 2024. PMID: 38834301 Free PMC article.
-
Psychophysical measurement of perceived motion flow of naturalistic scenes.iScience. 2023 Oct 23;26(12):108307. doi: 10.1016/j.isci.2023.108307. eCollection 2023 Dec 15. iScience. 2023. PMID: 38025782 Free PMC article.
-
Probabilistically constrained vector summation of motion direction in the mouse superior colliculus.Curr Biol. 2025 Feb 24;35(4):723-733.e3. doi: 10.1016/j.cub.2024.12.029. Epub 2025 Jan 21. Curr Biol. 2025. PMID: 39842438
-
Pattern Motion Direction Is Encoded in the Population Activity of Macaque Area MT.J Neurosci. 2022 Dec 14;42(50):9372-9386. doi: 10.1523/JNEUROSCI.0011-22.2022. Epub 2022 Nov 4. J Neurosci. 2022. PMID: 36332976 Free PMC article.
References
-
- Adelson EH, Movshon JA. The perception of coherent motion in two-dimensional patterns. Proceedings of the Association of Computing Machinery Interdisciplinary Workshop on Motion: Representation and Perception. Toronto, Canada, April 4–6, 1983, p. 11–14.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

