Inhibition of spindle extension through the yeast S phase checkpoint is coupled to replication fork stability and the integrity of centromeric DNA
- PMID: 31509480
- PMCID: PMC6789157
- DOI: 10.1091/mbc.E19-03-0156
Inhibition of spindle extension through the yeast S phase checkpoint is coupled to replication fork stability and the integrity of centromeric DNA
Abstract
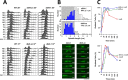
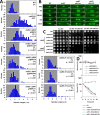
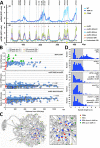
Budding yeast treated with hydroxyurea (HU) activate the S phase checkpoint kinase Rad53, which prevents DNA replication forks from undergoing aberrant structural transitions and nuclease processing. Rad53 is also required to prevent premature extension of the mitotic spindle that assembles during a HU-extended S phase. Here we present evidence that checkpoint restraint of spindle extension is directly coupled to Rad53 control of replication fork stability. In budding yeast, centromeres are flanked by replication origins that fire in early S phase. Mutations affecting the Zn2+-finger of Dbf4, an origin activator, preferentially reduce centromere-proximal origin firing in HU, corresponding with suppression of rad53 spindle extension. Inactivating Exo1 nuclease or displacing centromeres from origins provides a similar suppression. Conversely, short-circuiting Rad53 targeting of Dbf4, Sld3, and Dun1, substrates contributing to fork stability, induces spindle extension. These results reveal spindle extension in HU-treated rad53 mutants is a consequence of replication fork catastrophes at centromeres. When such catastrophes occur, centromeres become susceptible to nucleases, disrupting kinetochore function and spindle force balancing mechanisms. At the same time, our data indicate centromere duplication is not required to stabilize S phase spindle structure, leading us to propose a model for how monopolar kinetochore-spindle attachments may contribute to spindle force balance in HU.
Figures





Similar articles
-
The yeast S phase checkpoint enables replicating chromosomes to bi-orient and restrain spindle extension during S phase distress.J Cell Biol. 2005 Mar 28;168(7):999-1012. doi: 10.1083/jcb.200412076. J Cell Biol. 2005. PMID: 15795314 Free PMC article.
-
Damage-induced phosphorylation of Sld3 is important to block late origin firing.Nature. 2010 Sep 23;467(7314):479-83. doi: 10.1038/nature09377. Nature. 2010. PMID: 20865002 Free PMC article.
-
Centromere replication timing determines different forms of genomic instability in Saccharomyces cerevisiae checkpoint mutants during replication stress.Genetics. 2009 Dec;183(4):1249-60. doi: 10.1534/genetics.109.107508. Epub 2009 Oct 5. Genetics. 2009. PMID: 19805819 Free PMC article.
-
Taming the spindle for containing the chromosomes.Cell Cycle. 2005 Mar;4(3):376-9. doi: 10.4161/cc.4.3.1537. Epub 2005 Mar 17. Cell Cycle. 2005. PMID: 15701971 Review.
-
The S-phase checkpoint: targeting the replication fork.Biol Cell. 2009 Aug 19;101(11):617-27. doi: 10.1042/BC20090053. Biol Cell. 2009. PMID: 19686094 Review.
Cited by
-
Yeast Stn1 promotes MCM to circumvent Rad53 control of the S phase checkpoint.Curr Genet. 2022 Apr;68(2):165-179. doi: 10.1007/s00294-022-01228-0. Epub 2022 Feb 12. Curr Genet. 2022. PMID: 35150303 Free PMC article.
-
Hydroxyurea-The Good, the Bad and the Ugly.Genes (Basel). 2021 Jul 19;12(7):1096. doi: 10.3390/genes12071096. Genes (Basel). 2021. PMID: 34356112 Free PMC article. Review.
-
Coupling DNA Replication and Spindle Function in Saccharomyces cerevisiae.Cells. 2021 Nov 30;10(12):3359. doi: 10.3390/cells10123359. Cells. 2021. PMID: 34943867 Free PMC article. Review.
-
The yeast Dbf4 Zn2+ finger domain suppresses single-stranded DNA at replication forks initiated from a subset of origins.Curr Genet. 2022 Apr;68(2):253-265. doi: 10.1007/s00294-022-01230-6. Epub 2022 Feb 11. Curr Genet. 2022. PMID: 35147742 Free PMC article.
References
-
- Agarwal R, Tang Z, Yu H, Cohen-Fix O. (2003). Two distinct pathways for inhibiting pds1 ubiquitination in response to DNA damage. J Biol Chem , 45027–45033. - PubMed
-
- Alcasabas AA, Osborn AJ, Bachant J, Hu F, Werler PJ, Bousset K, Furuya K, Diffley JF, Carr AM, Elledge SJ. (2001). Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat Cell Biol , 958–965. - PubMed
-
- Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. (1994). The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev , 2401–2415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

