Clinical evaluation and budget impact analysis of cervical cancer screening using cobas 4800 HPV screening technology in the public sector of South Africa
- PMID: 31509545
- PMCID: PMC6738657
- DOI: 10.1371/journal.pone.0221495
Clinical evaluation and budget impact analysis of cervical cancer screening using cobas 4800 HPV screening technology in the public sector of South Africa
Abstract
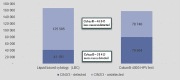
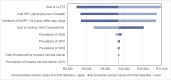
Cytology remains the mainstay of cervical cancer screening in South Africa (SA), however false negative rates are 25-50%. In contrast, human papillomavirus (HPV) screening techniques have higher sensitivity for cervical cancer precursors. The cobas® 4800 HPV test detects pooled high-risk HPV types and individual genotypes HPV 16 and 18. Using a mathematical budget impact model, the study objective was to evaluate the clinical and budget impact of replacing primary liquid-based cytology (LBC) with primary HPV-based screening strategies. In SA, current LBC screening practice recommends one test every ten years, followed by large loop excision of the transformation zone (LLETZ) if indicated. HPV testing can be performed from an LBC sample, where no additional consultations nor samples are required. In the budget impact model, LBC screening for 2 cycles (one test every ten years) was compared to cobas® 4800 HPV test for 2 cycles (one test every 5 years). The model inputs were gathered from literature and primary data sources. Indicative prices for LBC and cobas® 4800 HPV test were R189 and R457, respectively. Model results indicate that best outcomes for detection of disease were seen using cobas® 4800 HPV test. Forty-eight percent of cervical cancer cases were detected compared to 28% using LBC, and 50% of cervical intraepithelial neoplasia (CIN) 2 and CIN3 cases, compared to 25% with LBC. The budget impact analysis predicted that the cost per detected case of CIN2 or higher would be R 56,835 and R46,980 for the cobas® 4800 HPV and LBC scenarios, respectively. This equates to an incremental cost per detected case of CIN2 or higher of R9 855. From this model we conclude that a primary HPV screening strategy will have a significant clinical impact on disease burden in South Africa.
Conflict of interest statement
The authors’ affiliation with TCD Outcomes Research does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures






Similar articles
-
Detection Rate of High-Grade Cervical Neoplasia and Cost-Effectiveness of High-Risk Human Papillomavirus Genotyping with Reflex Liquid-based Cytology in Cervical Cancer Screening.Ann Acad Med Singap. 2017 Jul;46(7):267-273. Ann Acad Med Singap. 2017. PMID: 28821890
-
Budget impact analysis of cervical cancer screening in Portugal: comparison of cytology and primary HPV screening strategies.BMC Public Health. 2019 Feb 26;19(1):235. doi: 10.1186/s12889-019-6536-4. BMC Public Health. 2019. PMID: 30808324 Free PMC article.
-
Comparison of HPV-16 and HPV-18 Genotyping and Cytological Testing as Triage Testing Within Human Papillomavirus-Based Screening in Mexico.JAMA Netw Open. 2019 Nov 1;2(11):e1915781. doi: 10.1001/jamanetworkopen.2019.15781. JAMA Netw Open. 2019. PMID: 31747033 Free PMC article.
-
First international proficiency study on human papillomavirus testing in cervical cancer screening.J Clin Virol. 2023 Oct;167:105581. doi: 10.1016/j.jcv.2023.105581. Epub 2023 Sep 4. J Clin Virol. 2023. PMID: 37688950 Review.
-
Is 58% sensitivity for detection of cervical intraepithelial neoplasia 3 and invasive cervical cancer optimal for cervical screening?Cytojournal. 2014 May 22;11:14. doi: 10.4103/1742-6413.132997. eCollection 2014. Cytojournal. 2014. PMID: 24987445 Free PMC article. Review.
Cited by
-
The performance of Cobas HPV test for cervical cancer screening in Chinese female migrant workers.Epidemiol Infect. 2021 Aug 9;149:e196. doi: 10.1017/S0950268821001904. Epidemiol Infect. 2021. PMID: 34369328 Free PMC article.
-
Mathematical Models for Evaluating Effectiveness and Cost-Effectiveness of Cervical Cancer Control Policies in Populations Including Women Living With Human Immunodeficiency Virus: A Scoping Review.Value Health Reg Issues. 2022 Nov;32:39-46. doi: 10.1016/j.vhri.2022.07.001. Epub 2022 Sep 2. Value Health Reg Issues. 2022. PMID: 36063639 Free PMC article.
-
Cost-effectiveness of single-visit cervical cancer screening in KwaZulu-Natal, South Africa: a model-based analysis accounting for the HIV epidemic.Front Oncol. 2024 Apr 24;14:1382599. doi: 10.3389/fonc.2024.1382599. eCollection 2024. Front Oncol. 2024. PMID: 38720798 Free PMC article.
-
Immunocytochemical staining for p16, Ki-67, and MCM2 in the detection of cervical lesions and cancer: a prospective observational study.Transl Cancer Res. 2025 May 30;14(5):3201-3211. doi: 10.21037/tcr-2025-802. Epub 2025 May 27. Transl Cancer Res. 2025. PMID: 40530144 Free PMC article.
-
A community-based approach to cervical cancer prevention in western Kenya: An AMPATH feasibility project.SAGE Open Med. 2022 May 23;10:20503121221102111. doi: 10.1177/20503121221102111. eCollection 2022. SAGE Open Med. 2022. PMID: 35646368 Free PMC article.
References
-
- South African National Cancer Registry. Cancer in South Africa: 2012. [Internet], (2014) Johannesburg [Accessed 1 June 2019] Available from: http://www. nicd.ac.za/index.php/centres/national-cancer-registry/
-
- Dreyer G. (2017). General population screening study & VACCS2 GP. Unpublished raw data

