Neuropathy and neural plasticity in the subcutaneous white adipose depot
- PMID: 31509546
- PMCID: PMC6738614
- DOI: 10.1371/journal.pone.0221766
Neuropathy and neural plasticity in the subcutaneous white adipose depot
Abstract
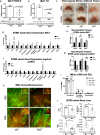
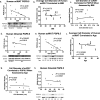
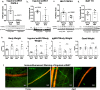
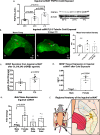
The difficulty in obtaining as well as maintaining weight loss, together with the impairment of metabolic control in conditions like diabetes and cardiovascular disease, may represent pathological situations of inadequate neural communication between the brain and peripheral organs and tissues. Innervation of adipose tissues by peripheral nerves provides a means of communication between the master metabolic regulator in the brain (chiefly the hypothalamus), and energy-expending and energy-storing cells in the body (primarily adipocytes). Although chemical and surgical denervation studies have clearly demonstrated how crucial adipose tissue neural innervation is for maintaining proper metabolic health, we have uncovered that adipose tissue becomes neuropathic (ie: reduction in neurites) in various conditions of metabolic dysregulation. Here, utilizing both human and mouse adipose tissues, we present evidence of adipose tissue neuropathy, or loss of proper innervation, under pathophysiological conditions such as obesity, diabetes, and aging, all of which are concomitant with insult to the adipose organ as well as metabolic dysfunction. Neuropathy is indicated by loss of nerve fiber protein expression, reduction in synaptic markers, and lower neurotrophic factor expression in adipose tissue. Aging-related adipose neuropathy particularly results in loss of innervation around the tissue vasculature, which cannot be reversed by exercise. Together with indications of neuropathy in muscle and bone, these findings underscore that peripheral neuropathy is not restricted to classic tissues like the skin of distal extremities, and that loss of innervation to adipose may trigger or exacerbate metabolic diseases. In addition, we have demonstrated stimulation of adipose tissue neural plasticity with cold exposure, which may ameliorate adipose neuropathy and be a potential therapeutic option to re-innervate adipose and restore metabolic health.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance.Biology (Basel). 2019 Feb 12;8(1):10. doi: 10.3390/biology8010010. Biology (Basel). 2019. PMID: 30759876 Free PMC article. Review.
-
Adipose Tissue Myeloid-Lineage Neuroimmune Cells Express Genes Important for Neural Plasticity and Regulate Adipose Innervation.Front Endocrinol (Lausanne). 2022 Jun 20;13:864925. doi: 10.3389/fendo.2022.864925. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35795142 Free PMC article.
-
Gene therapy approaches for obesity-induced adipose neuropathy: Device-targeted AAV-mediated neurotrophic factor delivery to adipocytes in subcutaneous adipose.Mol Ther. 2024 May 1;32(5):1407-1424. doi: 10.1016/j.ymthe.2024.02.035. Epub 2024 Mar 1. Mol Ther. 2024. PMID: 38429927 Free PMC article.
-
The involvement of neuroimmune cells in adipose innervation.Mol Med. 2020 Dec 9;26(1):126. doi: 10.1186/s10020-020-00254-3. Mol Med. 2020. PMID: 33297933 Free PMC article.
-
Obesity associated disease risk: the role of inherent differences and location of adipose depots.Horm Mol Biol Clin Investig. 2018 Mar 16;33(2):/j/hmbci.2018.33.issue-2/hmbci-2018-0012/hmbci-2018-0012.xml. doi: 10.1515/hmbci-2018-0012. Horm Mol Biol Clin Investig. 2018. PMID: 29547393 Review.
Cited by
-
Neuroendocrine Regulation of Energy Metabolism Involving Different Types of Adipose Tissues.Int J Mol Sci. 2019 Jun 1;20(11):2707. doi: 10.3390/ijms20112707. Int J Mol Sci. 2019. PMID: 31159462 Free PMC article. Review.
-
The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance.Biology (Basel). 2019 Feb 12;8(1):10. doi: 10.3390/biology8010010. Biology (Basel). 2019. PMID: 30759876 Free PMC article. Review.
-
Interactions between central nervous system and peripheral metabolic organs.Sci China Life Sci. 2022 Oct;65(10):1929-1958. doi: 10.1007/s11427-021-2103-5. Epub 2022 Jun 24. Sci China Life Sci. 2022. PMID: 35771484 Review.
-
Adipose tissue as a linchpin of organismal ageing.Nat Metab. 2024 May;6(5):793-807. doi: 10.1038/s42255-024-01046-3. Epub 2024 May 23. Nat Metab. 2024. PMID: 38783156 Free PMC article. Review.
-
Diet-induced loss of adipose hexokinase 2 correlates with hyperglycemia.Elife. 2023 Mar 15;12:e85103. doi: 10.7554/eLife.85103. Elife. 2023. PMID: 36920797 Free PMC article.
References
-
- Ryu V, Watts AG, Xue B, Bartness TJ. Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. American journal of physiology Regulatory, integrative and comparative physiology. 2017;312(3):R324–r37. Epub 2017/01/13. 10.1152/ajpregu.00456.2015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

