Distraction of olfactory bulb-medial prefrontal cortex circuit may induce anxiety-like behavior in allergic rhinitis
- PMID: 31509547
- PMCID: PMC6738655
- DOI: 10.1371/journal.pone.0221978
Distraction of olfactory bulb-medial prefrontal cortex circuit may induce anxiety-like behavior in allergic rhinitis
Abstract
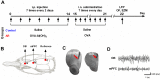
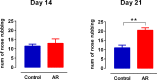
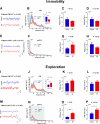
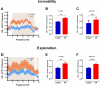
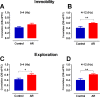
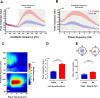
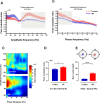
Allergic rhinitis is a chronic inflammatory disease of the upper respiratory tract, which is associated with high incidence of anxiety symptom. There is evidence that medial prefrontal cortex modulates anxiety-related behaviors and receives projections from olfactory bulb. Since olfactory dysfunction has been reported in allergic rhinitis, we aimed to evaluate anxiety-like behavior and oscillations of olfactory bulb-medial prefrontal cortex circuit in an animal model of allergic rhinitis. The number of open arm entries in elevated zero maze was significantly reduced in sensitized rats exposed to intranasal ovalbumin compared to the control group, which was indicating the enhancement of anxiety-like behavior in allergic rhinitis animals. Analysis of local field potentials in olfactory bulb and medial prefrontal cortex during immobility and exploration state showed that anxiety-like behavior induced by allergic rhinitis was in association with increased activity of medial prefrontal cortex and enhancement of olfactory bulb-medial prefrontal cortex coupling in delta and theta bands. Moreover, in allergic rhinitis animals, theta strongly coordinates local gamma activity in olfactory bulb and medial prefrontal cortex, which means to have a strong local theta/gamma coupling. We suggested that disruption of olfactory bulb-medial prefrontal cortex circuit due to allergic reactions might have a governing role for inducing anxiety-like behavior in the allergic rhinitis experimental model.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Olfactory bulb-medial prefrontal cortex theta synchronization is associated with anxiety.Sci Rep. 2024 May 27;14(1):12101. doi: 10.1038/s41598-024-63101-z. Sci Rep. 2024. PMID: 38802558 Free PMC article.
-
Structural, functional and behavioral impact of allergic rhinitis on olfactory pathway and prefrontal cortex.Physiol Behav. 2023 Jun 1;265:114171. doi: 10.1016/j.physbeh.2023.114171. Epub 2023 Mar 23. Physiol Behav. 2023. PMID: 36965572
-
Postnatal Allergic Inhalation Induces Glial Inflammation in the Olfactory Bulb and Leads to Autism-Like Traits in Mice.Int J Mol Sci. 2024 Sep 28;25(19):10464. doi: 10.3390/ijms251910464. Int J Mol Sci. 2024. PMID: 39408806 Free PMC article.
-
Allergen-induced anxiety-like behavior is associated with disruption of medial prefrontal cortex - amygdala circuit.Sci Rep. 2019 Dec 20;9(1):19586. doi: 10.1038/s41598-019-55539-3. Sci Rep. 2019. PMID: 31863052 Free PMC article.
-
Medial prefrontal cortex encoding of stress and anxiety.Int Rev Neurobiol. 2021;158:29-55. doi: 10.1016/bs.irn.2020.11.014. Epub 2021 Mar 19. Int Rev Neurobiol. 2021. PMID: 33785149 Review.
Cited by
-
High-fat diet feeding triggers a regenerative response in the adult zebrafish brain.Mol Neurobiol. 2023 May;60(5):2486-2506. doi: 10.1007/s12035-023-03210-4. Epub 2023 Jan 21. Mol Neurobiol. 2023. PMID: 36670270
-
Allergic Rhinitis and Depression: Profile and Proposal.Front Psychiatry. 2022 Jan 4;12:820497. doi: 10.3389/fpsyt.2021.820497. eCollection 2021. Front Psychiatry. 2022. PMID: 35058825 Free PMC article. Review.
-
Investigating Olfactory Sensory Neurons Facilitation For Aerobic Exercise-induced Spatial Memory Improvement.Basic Clin Neurosci. 2024 May-Jun;15(3):355-366. doi: 10.32598/bcn.2022.4029.1. Epub 2024 May 1. Basic Clin Neurosci. 2024. PMID: 39403352 Free PMC article.
-
Olfactory epithelium electrical stimulation mitigates memory and synaptic deficits caused by mechanical ventilation.Sci Rep. 2025 Apr 9;15(1):12197. doi: 10.1038/s41598-025-96661-9. Sci Rep. 2025. PMID: 40204831 Free PMC article.
-
Mechanism of allergic rhinitis treated by Centipeda minima from different geographic areas.Pharm Biol. 2021 Dec;59(1):606-618. doi: 10.1080/13880209.2021.1923757. Pharm Biol. 2021. PMID: 34010591 Free PMC article.
References
-
- Maurer M, Zuberbier T. Undertreatment of rhinitis symptoms in Europe: Findings from a cross-sectional questionnaire survey. Allergy Eur J Allergy Clin Immunol. 2007;62(9):1057–63. - PubMed
-
- Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma (ARIA)(ARIA Workshop Report). J Allergy Clin Immunol. 2001;108(5 Suppl):S147–334. - PubMed
-
- Bernstein DI, Schwartz G, Bernstein JA. Allergic rhinitis: mechanisms and treatment. Immunol Allergy Clin. 2016;36(2):261–78. - PubMed

