Amyloid β oligomers inhibit growth of human cancer cells
- PMID: 31509551
- PMCID: PMC6738617
- DOI: 10.1371/journal.pone.0221563
Amyloid β oligomers inhibit growth of human cancer cells
Abstract
Effects of amyloid beta (Aβ) oligomers on viability and function of cell lines such as NB4 (human acute promyelocytic leukemia), A549 (human lung cancer (adenocarcinomic alveolar basal epithelial tumor)) and MCF-7 (human breast cancer (invasive breast ductal carcinoma)) were investigated. Two types of Aβ oligomers were used in the study. The first type was produced in the presence of oligomerization inhibitor, hexafluoroisopropanol (HFIP). The second type of amyloids was assembled in the absence of the inhibitor. The first type preparation was predominantly populated with dimers and trimers, while the second type contained mostly pentadecamers. These amyloid species exhibited different secondary protein structure with considerable amount of antiparallel β sheet structural elements in HFIP oligomerized Aβ mixtures. The effect of the cell growth inhibition, which was stronger in the case of HFIP Aβ oligomers, was observed for all cell lines. Tests aiming at elucidating the effects of the amyloid species on cell cycles showed little differences between amyloid preparations. This prompts us to conclude that the effect on the cancer cell proliferation rate is less specific to the biological processes developing inside the cells during the proliferation. Therefore, cell growth inhibition may involve interactions with the peripheral parts of the cancer cells, such as a phospholipid membrane, and only in case of the NB4 cells, where accumulation of amyloid species inside the cells was detected, one may imply the opposite. In general, cancer cells were much less susceptible to the damaging effects of amyloid oligomers compared to earlier observations in mixed neuronal cell cultures.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

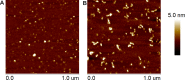
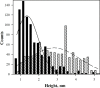
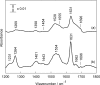






Similar articles
-
The alpha-to-beta conformational transition of Alzheimer's Abeta-(1-42) peptide in aqueous media is reversible: a step by step conformational analysis suggests the location of beta conformation seeding.Chembiochem. 2006 Feb;7(2):257-67. doi: 10.1002/cbic.200500223. Chembiochem. 2006. PMID: 16444756
-
Investigation on the influence of (Z)-3-(2-(3-chlorophenyl)hydrazono)-5,6-dihydroxyindolin-2-one (PT2) on β-amyloid(1-40) aggregation and toxicity.Arch Biochem Biophys. 2014 Oct 15;560:73-82. doi: 10.1016/j.abb.2014.07.015. Epub 2014 Jul 19. Arch Biochem Biophys. 2014. PMID: 25051344
-
Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer's disease.Sci Rep. 2018 Mar 19;8(1):4772. doi: 10.1038/s41598-018-23122-x. Sci Rep. 2018. PMID: 29555950 Free PMC article.
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
-
Conformation-specific antibodies to target amyloid β oligomers and their application to immunotherapy for Alzheimer's disease.Biosci Biotechnol Biochem. 2014;78(8):1293-305. doi: 10.1080/09168451.2014.940275. Biosci Biotechnol Biochem. 2014. PMID: 25130729 Review.
Cited by
-
Insights into Dysregulated Neurological Biomarkers in Cancer.Cancers (Basel). 2024 Jul 27;16(15):2680. doi: 10.3390/cancers16152680. Cancers (Basel). 2024. PMID: 39123408 Free PMC article. Review.
-
Distinguishing IDH mutation status in gliomas using FTIR-ATR spectra of peripheral blood plasma indicating clear traces of protein amyloid aggregation.BMC Cancer. 2024 Feb 16;24(1):222. doi: 10.1186/s12885-024-11970-y. BMC Cancer. 2024. PMID: 38365669 Free PMC article.
-
Insulin-Degrading Enzyme, an Under-Estimated Potential Target to Treat Cancer?Cells. 2022 Apr 5;11(7):1228. doi: 10.3390/cells11071228. Cells. 2022. PMID: 35406791 Free PMC article. Review.
-
Understanding human aging and the fundamental cell signaling link in age-related diseases: the middle-aging hypovascularity hypoxia hypothesis.Front Aging. 2023 Jun 13;4:1196648. doi: 10.3389/fragi.2023.1196648. eCollection 2023. Front Aging. 2023. PMID: 37384143 Free PMC article.
-
The Roles of the Amyloid Beta Monomers in Physiological and Pathological Conditions.Biomedicines. 2023 May 10;11(5):1411. doi: 10.3390/biomedicines11051411. Biomedicines. 2023. PMID: 37239082 Free PMC article. Review.
References
-
- Voytyuk I, De Strooper B, Chavez-Gutierrez L. Modulation of γ- and β-Secretases as Early Prevention Against Alzheimer's Disease. Biol. Psychiatry. 2018; 83: 320–327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

