A Midbrain Circuit that Mediates Headache Aversiveness in Rats
- PMID: 31509737
- PMCID: PMC6831085
- DOI: 10.1016/j.celrep.2019.08.009
A Midbrain Circuit that Mediates Headache Aversiveness in Rats
Abstract
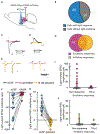
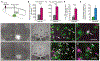
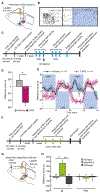
Migraines are a major health burden, but treatment is limited because of inadequate understanding of neural mechanisms underlying headache. Imaging studies of migraine patients demonstrate changes in both pain-modulatory circuits and reward-processing regions, but whether these changes contribute to the experience of headache is unknown. Here, we demonstrate a direct connection between the ventrolateral periaqueductal gray (vlPAG) and the ventral tegmental area (VTA) that contributes to headache aversiveness in rats. Many VTA neurons receive monosynaptic input from the vlPAG, and cranial nociceptive input increases Fos expression in VTA-projecting vlPAG neurons. Activation of PAG inputs to the VTA induces avoidance behavior, while inactivation of these projections induces a place preference only in animals with headache. This work identifies a distinct pathway that mediates cranial nociceptive aversiveness.
Keywords: headache; migraine; periaqueductal gray; ventral tegmental area; ventrolateral PAG.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

References
-
- Bandler R, and Depaulis A (1988). Elicitation of intraspecific defence reactions in the rat from midbrain periaqueductal grey by microinjection of kainic acid, without neurotoxic effects. Neurosci. Lett 88, 291–296. - PubMed
-
- Barbaresi P, and Manfrini E (1988). Glutamate decarboxylase-immunoreactive neurons and terminals in the periaqueductal gray of the rat. Neuroscience 27, 183–191. - PubMed
-
- Behbehani MM (1995). Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobiol 46, 575–605. - PubMed
-
- Behbehani MM, and Fields HL (1979). Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 170, 85–93. - PubMed
-
- Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, and Desai P (2018). Direct and Indirect Healthcare Resource Utilization and Costs Among Migraine Patients in the United States. Headache 58, 700–714. - PubMed

