Orphan Nuclear Receptor NR2F6 Suppresses T Follicular Helper Cell Accumulation through Regulation of IL-21
- PMID: 31509749
- PMCID: PMC6791812
- DOI: 10.1016/j.celrep.2019.08.024
Orphan Nuclear Receptor NR2F6 Suppresses T Follicular Helper Cell Accumulation through Regulation of IL-21
Abstract
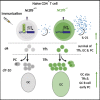
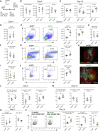
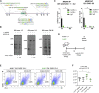
CD4 T follicular helper (Tfh) cells are specialized in helping B cells during the germinal center (GC) reaction and ultimately promote long-term humoral immunity. Here we report that loss of the nuclear orphan receptor NR2F6 causes enhanced survival and accumulation of Tfh cells, GC B cells, and plasma cells (PCs) following T cell-dependent immunization. Nr2f6-deficient CD4 T cell dysfunction is the primary cause of cell accumulation. Cytokine expression in Nr2f6-deficient Tfh cells is dysregulated, and Il21 expression is enhanced. Mechanistically, NR2F6 binds directly to the interleukin 21 (IL-21) promoter and a conserved noncoding sequence (CNS) near the Il21 gene in resting CD4+ T cells. During Tfh cell differentiation, this direct NR2F6 DNA interaction is abolished. Enhanced Tfh cell accumulation in Nr2f6-deficient mice can be reverted by blocking IL-21R signaling. Thus, NR2F6 is a critical negative regulator of IL-21 cytokine production in Tfh cells and prevents excessive Tfh cell accumulation.
Keywords: IL-21; T follicular helper cell; autoimmunity; germinal center reaction; immunization; nuclear receptor; systemic lupus erythematosus.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
IL-21 Receptor Blockade Shifts the Follicular T Cell Balance and Reduces De Novo Donor-Specific Antibody Generation.Front Immunol. 2021 Apr 9;12:661580. doi: 10.3389/fimmu.2021.661580. eCollection 2021. Front Immunol. 2021. PMID: 33897706 Free PMC article.
-
Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans.Blood. 2014 Jun 19;123(25):3988-98. doi: 10.1182/blood-2014-03-562231. Epub 2014 May 12. Blood. 2014. PMID: 24820310 Free PMC article.
-
Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages.Immunity. 2008 Jul 18;29(1):138-49. doi: 10.1016/j.immuni.2008.05.009. Epub 2008 Jul 3. Immunity. 2008. PMID: 18599325 Free PMC article.
-
T follicular helper (Tfh ) cells in normal immune responses and in allergic disorders.Allergy. 2016 Aug;71(8):1086-94. doi: 10.1111/all.12878. Epub 2016 May 25. Allergy. 2016. PMID: 26970097 Review.
-
Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses.Front Immunol. 2018 Oct 22;9:2412. doi: 10.3389/fimmu.2018.02412. eCollection 2018. Front Immunol. 2018. PMID: 30405612 Free PMC article. Review.
Cited by
-
Regulation of the germinal center response by nuclear receptors and implications for autoimmune diseases.FEBS J. 2020 Jul;287(14):2866-2890. doi: 10.1111/febs.15312. Epub 2020 Apr 19. FEBS J. 2020. PMID: 32246891 Free PMC article. Review.
-
The genetics of resistance to infectious pancreatic necrosis virus in rainbow trout unveiled through survival and virus load data.Front Genet. 2024 Nov 19;15:1484287. doi: 10.3389/fgene.2024.1484287. eCollection 2024. Front Genet. 2024. PMID: 39628812 Free PMC article.
-
Emerging Next-Generation Target for Cancer Immunotherapy Research: The Orphan Nuclear Receptor NR2F6.Cancers (Basel). 2021 May 26;13(11):2600. doi: 10.3390/cancers13112600. Cancers (Basel). 2021. PMID: 34073258 Free PMC article. Review.
-
A role for the nuclear receptor NR2F6 in peritoneal B cell homeostasis.Front Immunol. 2022 Aug 16;13:845235. doi: 10.3389/fimmu.2022.845235. eCollection 2022. Front Immunol. 2022. PMID: 36052079 Free PMC article.
-
Abnormally high expression of D1-like dopamine receptors on lupus CD4+ T cells promotes Tfh cell differentiation.Lupus Sci Med. 2023 Aug;10(2):e000943. doi: 10.1136/lupus-2023-000943. Lupus Sci Med. 2023. PMID: 37586763 Free PMC article.
References
-
- Baumjohann D., Preite S., Reboldi A., Ronchi F., Ansel K.M., Lanzavecchia A., Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

