A Monte Carlo model for organ dose reconstruction of patients in pencil beam scanning (PBS) proton therapy for epidemiologic studies of late effects
- PMID: 31509813
- PMCID: PMC10065358
- DOI: 10.1088/1361-6498/ab437d
A Monte Carlo model for organ dose reconstruction of patients in pencil beam scanning (PBS) proton therapy for epidemiologic studies of late effects
Abstract
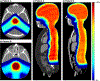
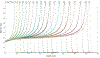
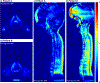
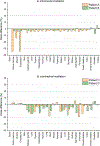
Significant efforts such as the Pediatric Proton/Photon Consortium Registry (PPCR) involving multiple proton therapy centers have been made to conduct collaborative studies evaluating outcomes following proton therapy. As a groundwork dosimetry effort for the late effect investigation, we developed a Monte Carlo (MC) model of proton pencil beam scanning (PBS) to estimate organ/tissue doses of pediatric patients at the Maryland Proton Treatment Center (MPTC), one of the proton centers involved in the PPCR. The MC beam modeling was performed using the TOPAS (TOol for PArticle Simulation) MC code and commissioned to match measurement data within 1% for range, and 0.3 mm for spot sizes. The established MC model was then tested by calculating organ/tissue doses for sample intracranial and craniospinal irradiations on whole-body pediatric computational human phantoms. The simulated dose distributions were compared with the treatment planning system dose distributions, showing the 3 mm/3% gamma index passing rates of 94%-99%, validating our simulations with the MC model. The calculated organ/tissue doses per prescribed doses for the craniospinal irradiations (1 mGy Gy-1 to 1 Gy Gy-1) were generally much higher than those for the intracranial irradiations (2.1 μGy Gy-1 to 0.1 Gy Gy-1), which is due to the larger field coverage of the craniospinal irradiations. The largest difference was observed at the adrenal dose, i.e. ∼3000 times. In addition, the calculated organ/tissue doses were compared with those calculated with a simplified MC model, showing that the beam properties (i.e. spot size, spot divergence, mean energy, and energy spread) do not significantly influence dose calculations despite the limited irradiation cases. This implies that the use of the MC model commissioned to the MPTC measurement data might be dosimetrically acceptable for patient dose reconstructions at other proton centers particularly when their measurement data are unavailable. The developed MC model will be used to reconstruct organ/tissue doses for MPTC pediatric patients collected in the PPCR.
Figures

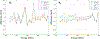


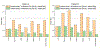
Similar articles
-
Fetal dose from proton pencil beam scanning craniospinal irradiation during pregnancy: a Monte Carlo study.Phys Med Biol. 2022 Jan 28;67(3):10.1088/1361-6560/ac4b38. doi: 10.1088/1361-6560/ac4b38. Phys Med Biol. 2022. PMID: 35026741 Free PMC article.
-
A dose voxel kernel method for rapid reconstruction of out-of-field neutron dose of patients in pencil beam scanning (PBS) proton therapy.Phys Med Biol. 2020 Aug 27;65(17):175015. doi: 10.1088/1361-6560/abaa5f. Phys Med Biol. 2020. PMID: 32726766 Free PMC article.
-
Validation of a Monte Carlo Framework for Out-of-Field Dose Calculations in Proton Therapy.Front Oncol. 2022 Jun 8;12:882489. doi: 10.3389/fonc.2022.882489. eCollection 2022. Front Oncol. 2022. PMID: 35756661 Free PMC article.
-
Validation and clinical implementation of an accurate Monte Carlo code for pencil beam scanning proton therapy.J Appl Clin Med Phys. 2018 Sep;19(5):558-572. doi: 10.1002/acm2.12420. Epub 2018 Jul 30. J Appl Clin Med Phys. 2018. PMID: 30058170 Free PMC article.
-
Monte Carlo simulation-based patient-specific QA using machine log files for line-scanning proton radiation therapy.Med Phys. 2023 Nov;50(11):7139-7153. doi: 10.1002/mp.16747. Epub 2023 Sep 27. Med Phys. 2023. PMID: 37756652
Cited by
-
Fetal dose from proton pencil beam scanning craniospinal irradiation during pregnancy: a Monte Carlo study.Phys Med Biol. 2022 Jan 28;67(3):10.1088/1361-6560/ac4b38. doi: 10.1088/1361-6560/ac4b38. Phys Med Biol. 2022. PMID: 35026741 Free PMC article.
-
A framework for in-field and out-of-field patient specific secondary cancer risk estimates from treatment plans using the TOPAS Monte Carlo system.Phys Med Biol. 2024 Aug 6;69(16):10.1088/1361-6560/ad64b6. doi: 10.1088/1361-6560/ad64b6. Phys Med Biol. 2024. PMID: 39019051 Free PMC article.
-
Risk of radiation-induced second malignant neoplasms from photon and proton radiotherapy in paediatric abdominal neuroblastoma.Phys Imaging Radiat Oncol. 2021 Jul 9;19:45-52. doi: 10.1016/j.phro.2021.06.003. eCollection 2021 Jul. Phys Imaging Radiat Oncol. 2021. PMID: 34307918 Free PMC article.
-
The Pediatric Proton and Photon Therapy Comparison Cohort: Study Design for a Multicenter Retrospective Cohort to Investigate Subsequent Cancers After Pediatric Radiation Therapy.Adv Radiat Oncol. 2023 May 21;8(6):101273. doi: 10.1016/j.adro.2023.101273. eCollection 2023 Nov-Dec. Adv Radiat Oncol. 2023. PMID: 38047226 Free PMC article.
-
A dose voxel kernel method for rapid reconstruction of out-of-field neutron dose of patients in pencil beam scanning (PBS) proton therapy.Phys Med Biol. 2020 Aug 27;65(17):175015. doi: 10.1088/1361-6560/abaa5f. Phys Med Biol. 2020. PMID: 32726766 Free PMC article.
References
-
- Allison J, Amako K, Apostolakis J, Arce P, Asai M, Aso T, Bagli E, Bagulya A, Banerjee S, Barrand G, Beck BR, Bogdanov AG, Brandt D, Brown JMC, Burkhardt H, Canal Ph, Cano-Ott D, Chauvie S, Cho K, Cirrone GAP, Cooperman G, Cortés-Giraldo MA, Cosmo G, Cuttone G, Depaola G, Desorgher L, Dong X, Dotti A, Elvira VD, Folger G, Francis Z, Galoyan A, Garnier L, Gayer M, Genser KL, Grichine VM, Guatelli S, Guèye P, Gumplinger P, Howard AS, Hfivnacova I, Hwang S, Incerti S, Ivanchenko A, Ivanchenko VN, Jones FW, Jun SY, Kaitaniemi P, Karakatsanis N, Karamitros M, Kelsey M, Kimura A, Koi T, Kurashige H, Lechner A, Lee SB, Longo F, Maire M, Mancusi D, Mantero A, Mendoza E, Morgan B, Murakami K, Nikitina T, Pandola L, Paprocki P, Perl J, Petrovic I, Pia MG, Pokorski W, Quesada JM, Raine M, Reis MA, Ribon A, Ristic Fira A, Romano F, Russo G, Santin G, Sasaki T, Sawkey D, Shin JI, Strakovsky II, Taborda A, Tanaka S, Tomé B, Toshito T, Tran HN, Truscott PR, Urban L, Uzhinsky V, Verbeke JM, Verderi M, Wendt BL, Wenzel H, Wright DH, Wright DM, Yamashita T, Yarba J, et al. 2016. Recent developments in Geant4 Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 835 186–225
-
- Ardenfors O, Gudowska I, Flejmer AM and Dasu A 2018. Impact of irradiation setup in proton spot scanning brain therapy on organ doses from secondary radiation Radiat Prot Dosimetry 180 261–6 - PubMed
-
- Berrington de Gonzalez A, Vikram B, Buchsbaum JC, de Vathaire F, Dörr W, Hass-Kogan D, Langendijk JA, Mahajan A, Newhauser W, Ottolenghi A, Ronckers C, Schulte R, Walsh L, Yock TI and Kleinerman RA 2017. A Clarion Call for Large-Scale Collaborative Studies of Pediatric Proton Therapy International Journal of Radiation Oncology*Biology*Physics 98 980–1 - PubMed
-
- Binns PJ and Hough JH 1997. Secondary Dose Exposures During 200 MeV Proton Therapy Radiat Prot Dosimetry 70 441–4
-
- Brenner DJ and Hall EJ 2008. Secondary neutrons in clinical proton radiotherapy: a charged issue. Radiotherapy and Oncology 86 165–70 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
