The Molecular Links between Cell Death and Inflammasome
- PMID: 31509938
- PMCID: PMC6769855
- DOI: 10.3390/cells8091057
The Molecular Links between Cell Death and Inflammasome
Abstract
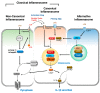
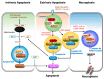
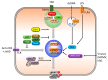
Programmed cell death pathways and inflammasome activation pathways can be genetically and functionally separated. Inflammasomes are specialized protein complexes that process pro-inflammatory cytokines, interleukin-1β (IL-1β), and IL-18 to bioactive forms for protection from a wide range of pathogens, as well as environmental and host-derived danger molecules. Programmed cell death has been extensively studied, and its role in the development, homeostasis, and control of infection and danger is widely appreciated. Apoptosis and the recently recognized necroptosis are the best-characterized forms of programmed death, and the interplay between them through death receptor signaling is also being studied. Moreover, growing evidence suggests that many of the signaling molecules known to regulate programmed cell death can also modulate inflammasome activation in a cell-intrinsic manner. Therefore, in this review, we will discuss the current knowledge concerning the role of the signaling molecules originally associated with programmed cell death in the activation of inflammasome and IL-1β processing.
Keywords: Caspase-8; DRP1; MLKL; PGAM5; RIPK1/3; apoptosis; inflammasome; necroptosis; programmed cell death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The intersection of cell death and inflammasome activation.Cell Mol Life Sci. 2016 Jun;73(11-12):2349-67. doi: 10.1007/s00018-016-2205-2. Epub 2016 Apr 11. Cell Mol Life Sci. 2016. PMID: 27066895 Free PMC article. Review.
-
The Mitochondrial Phosphatase PGAM5 Is Dispensable for Necroptosis but Promotes Inflammasome Activation in Macrophages.J Immunol. 2016 Jan 1;196(1):407-15. doi: 10.4049/jimmunol.1501662. Epub 2015 Nov 18. J Immunol. 2016. PMID: 26582950 Free PMC article.
-
MLKL-Driven Inflammasome Activation and Caspase-8 Mediate Inflammatory Cell Death in Influenza A Virus Infection.mBio. 2023 Apr 25;14(2):e0011023. doi: 10.1128/mbio.00110-23. Epub 2023 Feb 28. mBio. 2023. PMID: 36852999 Free PMC article.
-
The NS1 Protein of Influenza A Virus Participates in Necroptosis by Interacting with MLKL and Increasing Its Oligomerization and Membrane Translocation.J Virol. 2019 Jan 4;93(2):e01835-18. doi: 10.1128/JVI.01835-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355688 Free PMC article.
-
Caspase-8 functions as a key mediator of inflammation and pro-IL-1β processing via both canonical and non-canonical pathways.Immunol Rev. 2015 May;265(1):181-93. doi: 10.1111/imr.12284. Immunol Rev. 2015. PMID: 25879293 Review.
Cited by
-
Cross Kingdom Immunity: The Role of Immune Receptors and Downstream Signaling in Animal and Plant Cell Death.Front Immunol. 2021 Mar 8;11:612452. doi: 10.3389/fimmu.2020.612452. eCollection 2020. Front Immunol. 2021. PMID: 33763054 Free PMC article. Review.
-
Leptin Induces Apoptotic and Pyroptotic Cell Death via NLRP3 Inflammasome Activation in Rat Hepatocytes.Int J Mol Sci. 2021 Nov 22;22(22):12589. doi: 10.3390/ijms222212589. Int J Mol Sci. 2021. PMID: 34830465 Free PMC article.
-
Lipid-Sensing Receptor FFAR4 Modulates Pulmonary Epithelial Homeostasis following Immunogenic Exposures Independently of the FFAR4 Ligand Docosahexaenoic Acid (DHA).Int J Mol Sci. 2023 Apr 11;24(8):7072. doi: 10.3390/ijms24087072. Int J Mol Sci. 2023. PMID: 37108233 Free PMC article.
-
Role of Pyroptosis in Diabetes and Its Therapeutic Implications.J Inflamm Res. 2021 May 24;14:2187-2206. doi: 10.2147/JIR.S291453. eCollection 2021. J Inflamm Res. 2021. PMID: 34079327 Free PMC article. Review.
-
The Role of Natural Compounds and their Nanocarriers in the Treatment of CNS Inflammation.Biomolecules. 2020 Oct 1;10(10):1401. doi: 10.3390/biom10101401. Biomolecules. 2020. PMID: 33019651 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

