Site Selective Antibody-Oligonucleotide Conjugation via Microbial Transglutaminase
- PMID: 31509944
- PMCID: PMC6767100
- DOI: 10.3390/molecules24183287
Site Selective Antibody-Oligonucleotide Conjugation via Microbial Transglutaminase
Abstract
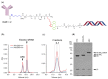
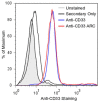
Nucleic Acid Therapeutics (NATs), including siRNAs and AntiSense Oligonucleotides (ASOs), have great potential to drug the undruggable genome. Targeting siRNAs and ASOs to specific cell types of interest has driven dramatic improvement in efficacy and reduction in toxicity. Indeed, conjugation of tris-GalNAc to siRNAs and ASOs has shown clinical efficacy in targeting diseases driven by liver hepatocytes. However, targeting non-hepatic diseases with oligonucleotide therapeutics has remained problematic for several reasons, including targeting specific cell types and endosomal escape. Monoclonal antibody (mAb) targeting of siRNAs and ASOs has the potential to deliver these drugs to a variety of specific cell and tissue types. However, most conjugation strategies rely on random chemical conjugation through lysine or cysteine residues resulting in conjugate heterogeneity and a distribution of Drug:Antibody Ratios (DAR). To produce homogeneous DAR-2 conjugates with two siRNAs per mAb, we developed a novel two-step conjugation procedure involving microbial transglutaminase (MTGase) tagging of the antibody C-terminus with an azide-functionalized linker peptide that can be subsequently conjugated to dibenzylcyclooctyne (DBCO) bearing oligonucleotides through azide-alkyne cycloaddition. Antibody-siRNA (and ASO) conjugates (ARCs) produced using this strategy are soluble, chemically defined targeted oligonucleotide therapeutics that have the potential to greatly increase the number of targetable cell types.
Keywords: antibody-siRNA conjugate (ARC); antisense oligonucleotides; copper-less click; microbial transglutaminase; monoclonal antibodies; oligonucleotide therapeutics; siRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Transglutaminase-based chemo-enzymatic conjugation approach yields homogeneous antibody-drug conjugates.Bioconjug Chem. 2014 Mar 19;25(3):569-78. doi: 10.1021/bc400574z. Epub 2014 Feb 12. Bioconjug Chem. 2014. PMID: 24483299
-
Conjugation of peptides to antisense interleukin-6 via click chemistry.Curr Med Chem. 2014 Apr;21(10):1247-54. doi: 10.2174/0929867320666131119125045. Curr Med Chem. 2014. PMID: 24251570
-
Structure-Activity Relationship of Antibody-Oligonucleotide Conjugates: Evaluating Bioconjugation Strategies for Antibody-siRNA Conjugates for Drug Development.J Med Chem. 2024 Sep 12;67(17):14852-14867. doi: 10.1021/acs.jmedchem.4c00802. Epub 2024 Aug 28. J Med Chem. 2024. PMID: 39197831 Free PMC article.
-
Metabolic Stability and Targeted Delivery of Oligonucleotides: Advancing RNA Therapeutics Beyond The Liver.J Med Chem. 2025 Apr 10;68(7):6870-6896. doi: 10.1021/acs.jmedchem.4c02528. Epub 2025 Jan 8. J Med Chem. 2025. PMID: 39772535 Free PMC article. Review.
-
Antibody-siRNA conjugates: drugging the undruggable for anti-leukemic therapy.Expert Opin Biol Ther. 2017 Mar;17(3):325-338. doi: 10.1080/14712598.2017.1273344. Epub 2016 Dec 23. Expert Opin Biol Ther. 2017. PMID: 27977315 Review.
Cited by
-
Antibody-Oligonucleotide Conjugates: A Twist to Antibody-Drug Conjugates.J Clin Med. 2021 Feb 18;10(4):838. doi: 10.3390/jcm10040838. J Clin Med. 2021. PMID: 33670689 Free PMC article. Review.
-
Transglutaminase in Foods and Biotechnology.Int J Mol Sci. 2023 Aug 3;24(15):12402. doi: 10.3390/ijms241512402. Int J Mol Sci. 2023. PMID: 37569776 Free PMC article. Review.
-
Generation and validation of structurally defined antibody-siRNA conjugates.Nucleic Acids Res. 2020 Jun 4;48(10):5281-5293. doi: 10.1093/nar/gkaa286. Nucleic Acids Res. 2020. PMID: 32347936 Free PMC article.
-
Noncoding RNA therapeutics - challenges and potential solutions.Nat Rev Drug Discov. 2021 Aug;20(8):629-651. doi: 10.1038/s41573-021-00219-z. Epub 2021 Jun 18. Nat Rev Drug Discov. 2021. PMID: 34145432 Free PMC article. Review.
-
Enzymatic Bioconjugation: A Perspective from the Pharmaceutical Industry.JACS Au. 2023 May 4;3(5):1267-1283. doi: 10.1021/jacsau.2c00617. eCollection 2023 May 22. JACS Au. 2023. PMID: 37234110 Free PMC article. Review.
References
-
- Taubel J., Zimmermann T., Karsten V., Martinez C., Chan A., Wang Y., Attarwala H., Gollob J., Vest J. Phase 1 Study of ALN-TTRsc02, a Subcutaneously Administered Investigational RNAi Therapeutic for the Treatment of Transthyretin-Mediated Amyloidosis. [(accessed on 29 July 2019)];2018 Available online: http://alnylam.com/wp-content/uploads/2018/03/10.-TTR-SCO2_FINAL.pdf.
-
- Balwani M., Gouya L., Rees D., Stein P., Stölzel U., Aguilera P., Bissell D.M., Bonkovsky H., Keel S., Parker C., et al. ENVISION, a Phase 3 Study to Evaluate the Efficacy and Safety of Givosiran, an Investigational RNAi Therapeutic Targeting Aminolevulinic Acid Synthase 1, in Acute Hepatic Porphyria Patients. [(accessed on 29 July 2019)];2019 Available online: https://www.alnylam.com/wp-content/uploads/2019/04/Balwani_ENVISION_EASL....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

