Site Selective Antibody-Oligonucleotide Conjugation via Microbial Transglutaminase
- PMID: 31509944
- PMCID: PMC6767100
- DOI: 10.3390/molecules24183287
Site Selective Antibody-Oligonucleotide Conjugation via Microbial Transglutaminase
Abstract
Nucleic Acid Therapeutics (NATs), including siRNAs and AntiSense Oligonucleotides (ASOs), have great potential to drug the undruggable genome. Targeting siRNAs and ASOs to specific cell types of interest has driven dramatic improvement in efficacy and reduction in toxicity. Indeed, conjugation of tris-GalNAc to siRNAs and ASOs has shown clinical efficacy in targeting diseases driven by liver hepatocytes. However, targeting non-hepatic diseases with oligonucleotide therapeutics has remained problematic for several reasons, including targeting specific cell types and endosomal escape. Monoclonal antibody (mAb) targeting of siRNAs and ASOs has the potential to deliver these drugs to a variety of specific cell and tissue types. However, most conjugation strategies rely on random chemical conjugation through lysine or cysteine residues resulting in conjugate heterogeneity and a distribution of Drug:Antibody Ratios (DAR). To produce homogeneous DAR-2 conjugates with two siRNAs per mAb, we developed a novel two-step conjugation procedure involving microbial transglutaminase (MTGase) tagging of the antibody C-terminus with an azide-functionalized linker peptide that can be subsequently conjugated to dibenzylcyclooctyne (DBCO) bearing oligonucleotides through azide-alkyne cycloaddition. Antibody-siRNA (and ASO) conjugates (ARCs) produced using this strategy are soluble, chemically defined targeted oligonucleotide therapeutics that have the potential to greatly increase the number of targetable cell types.
Keywords: antibody-siRNA conjugate (ARC); antisense oligonucleotides; copper-less click; microbial transglutaminase; monoclonal antibodies; oligonucleotide therapeutics; siRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


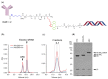
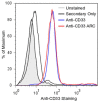
References
-
- Taubel J., Zimmermann T., Karsten V., Martinez C., Chan A., Wang Y., Attarwala H., Gollob J., Vest J. Phase 1 Study of ALN-TTRsc02, a Subcutaneously Administered Investigational RNAi Therapeutic for the Treatment of Transthyretin-Mediated Amyloidosis. [(accessed on 29 July 2019)];2018 Available online: http://alnylam.com/wp-content/uploads/2018/03/10.-TTR-SCO2_FINAL.pdf.
-
- Balwani M., Gouya L., Rees D., Stein P., Stölzel U., Aguilera P., Bissell D.M., Bonkovsky H., Keel S., Parker C., et al. ENVISION, a Phase 3 Study to Evaluate the Efficacy and Safety of Givosiran, an Investigational RNAi Therapeutic Targeting Aminolevulinic Acid Synthase 1, in Acute Hepatic Porphyria Patients. [(accessed on 29 July 2019)];2019 Available online: https://www.alnylam.com/wp-content/uploads/2019/04/Balwani_ENVISION_EASL....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

