Effect of Repeated Consumption of Partially Hydrolyzed Guar Gum on Fecal Characteristics and Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Clinical Trial
- PMID: 31509971
- PMCID: PMC6769658
- DOI: 10.3390/nu11092170
Effect of Repeated Consumption of Partially Hydrolyzed Guar Gum on Fecal Characteristics and Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Clinical Trial
Abstract
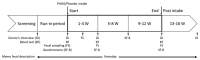
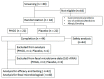
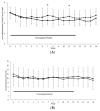
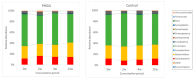
Partially hydrolyzed guar gum (PHGG) is a water-soluble dietary fiber and is used in solid and liquid food to regulate gut function. The aim of this study was to investigate effects of PHGG on bowel movements (stool form and frequency), plasma bile acids, quality of life, and gut microbiota of healthy volunteers with a tendency toward diarrhea, i.e., irritable bowel syndrome diarrhea (IBS-D)-like symptoms. A randomized, double-blind, placebo-controlled, and parallel trial was performed on 44 healthy volunteers (22 males, 22 females, 41.9 ± 6.3 years old (average ± SD)) with minimum 7 bowel movements every week, wherein above 50% of their stool was between the Bristol stool scale (BSS) value of 5 and 6. Intake of the PHGG for 3 months significantly improved stool form, evaluated using BSS, and had no effects on stool frequency. BSS was significantly normalized in the group consuming the PHGG compared with the placebo. Comprehensive fecal microbiome analysis by the 16S rRNA-sequence method detected significant changes in the ratio of some bacteria, such as an increase of Bifidobacterium (p < 0.05) in the PHGG group. Our results suggest that intake of PHGG improves human stool form via regulating intestinal microbiota.
Keywords: 16S rRNA; Bristol stool scale; diarrhea; gut microbiota; partially hydrolyzed guar gum.
Conflict of interest statement
Zenta Yasukawa and Makoto Ozeki and Tsutomu Okubo are employees of Taiyo Kagaku. Yuji Naito received scholarship fund from EA Pharma. Co., Ltd. and collaboration research fund from Fujifilm Medical Co., Ltd. and Taiyo Kagaku Co., Ltd., and has been paid lecture fees by Mylan EPD Co., Takeda Pharma. Co., Ltd., Mochida Pharma. Co., Ltd., EA Pharma. Co., Ltd., Otsuka Pharma. Co., Ltd., Nippon Kayaku Co., Ltd., and Miyarisan Pharma. Co., Ltd. The present research was partly funded by these funds. Neither the funding agency nor any outside organization has participated in study design or have any competing of interest. These companies had final approval of the manuscript.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

