Brain Delivery of a Potent Opioid Receptor Agonist, Biphalin during Ischemic Stroke: Role of Organic Anion Transporting Polypeptide (OATP)
- PMID: 31509975
- PMCID: PMC6781285
- DOI: 10.3390/pharmaceutics11090467
Brain Delivery of a Potent Opioid Receptor Agonist, Biphalin during Ischemic Stroke: Role of Organic Anion Transporting Polypeptide (OATP)
Abstract
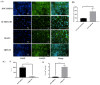
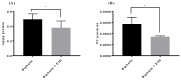
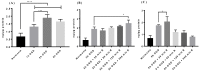
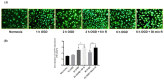
Transporters (expressed) at the blood-brain barrier (BBB) can play an essential role in the treatment of brain injury by transporting neuroprotective substance to the central nervous system. The goal of this study was to understand the role of organic anion transporting polypeptide (OATP1; OATP1A2 in humans and oatp1a4 in rodents) in the transport of a potent opioid receptor agonist, biphalin, across the BBB during ischemic stroke. Brain microvascular endothelial cells (BMECs) that were differentiated from human induced pluripotent stem cells (iPSCs) were used in the present study. The effect of oxygen-glucose deprivation (OGD) and reperfusion on the OATP1 expression, uptake, and transport of biphalin was measured in induced pluripotent stem cells differentiated brain microvascular endothelial cells (iPSC-BMECs) in the presence and absence of an OATP1 substrate, estrone-3-sulfate (E3S). Biphalin brain permeability was quantified while using a highly sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. It was found that iPSC-BMECs expressed OATP1. In vitro studies showed that biphalin BBB uptake and transport decreased in the presence of an OATP1 specific substrate. It was also observed that OGD and reperfusion modulate the expression and function of OATP1 in BMECs. This study strongly demonstrates that OATP1 contributes to the transport of biphalin across the BBB and increased expression of OATP1 during OGD-reperfusion could provide a novel target for improving ischemic brain drug delivery of biphalin or other potential neurotherapeutics that have affinity to this BBB transporter.
Keywords: biphalin; blood-brain barrier; ischemic stroke; organic anion transporting polypeptide; transport mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Glutamate Buffering Capacity and Blood-Brain Barrier Protection of Opioid Receptor Agonists Biphalin and Nociceptin.J Pharmacol Exp Ther. 2021 Nov;379(3):260-269. doi: 10.1124/jpet.121.000831. Epub 2021 Oct 18. J Pharmacol Exp Ther. 2021. PMID: 34663677
-
Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier.J Pharmacol Exp Ther. 2000 Jul;294(1):73-9. J Pharmacol Exp Ther. 2000. PMID: 10871297
-
Functional Investigation of Solute Carrier Family 35, Member F2, in Three Cellular Models of the Primate Blood-Brain Barrier.Drug Metab Dispos. 2021 Jan;49(1):3-11. doi: 10.1124/dmd.120.000115. Epub 2020 Nov 3. Drug Metab Dispos. 2021. PMID: 33144341
-
Functional Expression of P-glycoprotein and Organic Anion Transporting Polypeptides at the Blood-Brain Barrier: Understanding Transport Mechanisms for Improved CNS Drug Delivery?AAPS J. 2017 Jul;19(4):931-939. doi: 10.1208/s12248-017-0081-9. Epub 2017 Apr 26. AAPS J. 2017. PMID: 28447295 Free PMC article. Review.
-
Pharmacogenetics of the organic anion transporting polypeptide 1A2.Pharmacogenomics. 2009 Mar;10(3):339-44. doi: 10.2217/14622416.10.3.339. Pharmacogenomics. 2009. PMID: 19290786 Free PMC article. Review.
Cited by
-
Transport Properties of Statins by Organic Anion Transporting Polypeptide 1A2 and Regulation by Transforming Growth Factor-β Signaling in Human Endothelial Cells.J Pharmacol Exp Ther. 2021 Feb;376(2):148-160. doi: 10.1124/jpet.120.000267. Epub 2020 Nov 9. J Pharmacol Exp Ther. 2021. PMID: 33168642 Free PMC article.
-
Modeling blood-brain barrier pathology in cerebrovascular disease in vitro: current and future paradigms.Fluids Barriers CNS. 2020 Jul 16;17(1):44. doi: 10.1186/s12987-020-00202-7. Fluids Barriers CNS. 2020. PMID: 32677965 Free PMC article. Review.
-
Transport Mechanisms at the Blood-Brain Barrier and in Cellular Compartments of the Neurovascular Unit: Focus on CNS Delivery of Small Molecule Drugs.Pharmaceutics. 2022 Jul 20;14(7):1501. doi: 10.3390/pharmaceutics14071501. Pharmaceutics. 2022. PMID: 35890396 Free PMC article. Review.
-
Role of Resolvins in the Inflammatory Resolution of Neurological Diseases.Front Pharmacol. 2020 May 8;11:612. doi: 10.3389/fphar.2020.00612. eCollection 2020. Front Pharmacol. 2020. PMID: 32457616 Free PMC article. Review.
-
Blood-Brain Barrier Transporters: Opportunities for Therapeutic Development in Ischemic Stroke.Int J Mol Sci. 2022 Feb 8;23(3):1898. doi: 10.3390/ijms23031898. Int J Mol Sci. 2022. PMID: 35163820 Free PMC article. Review.
References
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

