A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability
- PMID: 31510010
- PMCID: PMC6770861
- DOI: 10.3390/ijms20184459
A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability
Abstract
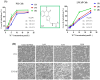
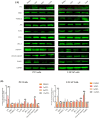
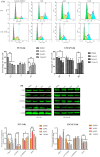
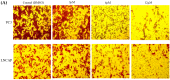
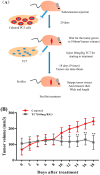
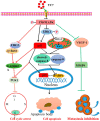
Prostate cancer (PCa), an epithelial malignant tumor, is the second common cause of cancer death among males in western countries. Thus, the development of new strategies is urgently needed. Tanshinones isolated from Salvia miltiorrhiza and its synthetic analogs show various biological activities including anticancer effects. Among them, the tanshinone analog 2-((Glycine methyl ester)methyl)-naphtho (TC7) is the most effective, with better selectivity and lower toxicity. Therefore, in this work, the effect of TC7 against PCa was investigated through assessing the molecular mechanisms regulating the growth, metastasis, and invasion of PCa cells. Human PCa cells, PC3 and LNCAP, were used to evaluate TC7 mechanisms of action in vitro, while male BALB/c nude mice were used for in vivo experiments by subjecting each mouse to a subcutaneous injection of PC3 cells into the right flank to evaluate TC7 effects on tumor volume. Our in vitro results showed that TC7 inhibited cell proliferation by arresting the cell cycle at G2/M through the regulation of cyclin b1, p53, GADD45A, PLK1, and CDC2/cyclin b1. In addition, TC7 induced cell apoptosis by regulating apoptosis-associated genes such as p53, ERK1, BAX, p38, BCL-2, caspase-8, cleaved-caspase-8, PARP1, and the phosphorylation level of ERK1 and p38. Furthermore, it decreased DNA synthesis and inhibited the migration and invasion ability by regulating VEGF-1 and MMP-9 protein expression. Our in vivo evidence supports the conclusion that TC7 could be considered as a potential promising chemotherapeutic candidate in the treatment of PCa.
Keywords: cell apoptosis; cell cycle arrest; metastasis potency; prostate cancer; quinone analog.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Tanshinones from Chinese medicinal herb Danshen (Salvia miltiorrhiza Bunge) suppress prostate cancer growth and androgen receptor signaling.Pharm Res. 2012 Jun;29(6):1595-608. doi: 10.1007/s11095-012-0670-3. Epub 2012 Jan 27. Pharm Res. 2012. PMID: 22281759
-
Apoptosis induced by the methanol extract of Salvia miltiorrhiza Bunge in non-small cell lung cancer through PTEN-mediated inhibition of PI3K/Akt pathway.J Ethnopharmacol. 2017 Mar 22;200:107-116. doi: 10.1016/j.jep.2016.12.051. Epub 2017 Jan 12. J Ethnopharmacol. 2017. PMID: 28088493
-
Evaluation of the anticancer and anti-metastasis effects of novel synthetic sodium channel blockers in prostate cancer cells in vitro and in vivo.Prostate. 2019 Jan;79(1):62-72. doi: 10.1002/pros.23711. Epub 2018 Sep 21. Prostate. 2019. PMID: 30242862
-
Pharmacological properties of tanshinones, the natural products from Salvia miltiorrhiza.Adv Pharmacol. 2020;87:43-70. doi: 10.1016/bs.apha.2019.10.001. Epub 2020 Jan 13. Adv Pharmacol. 2020. PMID: 32089238 Review.
-
Tanshinones: sources, pharmacokinetics and anti-cancer activities.Int J Mol Sci. 2012 Oct 22;13(10):13621-66. doi: 10.3390/ijms131013621. Int J Mol Sci. 2012. PMID: 23202971 Free PMC article. Review.
Cited by
-
Discovery of novel quinazoline derivatives containing trifluoromethyl against cell proliferation by targeting werner helicase.Mol Divers. 2025 Mar 28. doi: 10.1007/s11030-025-11175-w. Online ahead of print. Mol Divers. 2025. PMID: 40148606
-
Galactose-Modified PH-Sensitive Niosomes for Controlled Release and Hepatocellular Carcinoma Target Delivery of Tanshinone IIA.AAPS PharmSciTech. 2021 Mar 10;22(3):96. doi: 10.1208/s12249-021-01973-4. AAPS PharmSciTech. 2021. PMID: 33694067 Free PMC article.
-
Tanshinone IIA induces autophagy in colon cancer cells through MEK/ERK/mTOR pathway.Transl Cancer Res. 2020 Nov;9(11):6919-6928. doi: 10.21037/tcr-20-1963. Transl Cancer Res. 2020. PMID: 35117300 Free PMC article.
-
Quercetin and Its Nano-Scale Delivery Systems in Prostate Cancer Therapy: Paving the Way for Cancer Elimination and Reversing Chemoresistance.Cancers (Basel). 2021 Mar 31;13(7):1602. doi: 10.3390/cancers13071602. Cancers (Basel). 2021. PMID: 33807174 Free PMC article. Review.
-
MicroRNA-92a Targets SERTAD3 and Regulates the Growth, Invasion, and Migration of Prostate Cancer Cells via the P53 Pathway.Onco Targets Ther. 2020 Jun 12;13:5495-5514. doi: 10.2147/OTT.S249168. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606766 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

