Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells-A Concise Review
- PMID: 31510023
- PMCID: PMC6781041
- DOI: 10.3390/nano9091292
Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells-A Concise Review
Abstract
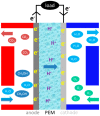
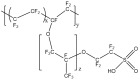
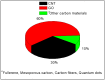
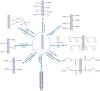
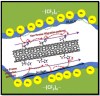
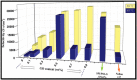
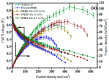
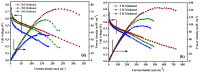
A membrane electrolyte that restricts the methanol cross-over while retaining proton conductivity is essential for better electrochemical selectivity in direct methanol fuel cells (DMFCs). Extensive research carried out to explore numerous blends and composites for application as polymer electrolyte membranes (PEMs) revealed promising electrochemical selectivity in DMFCs of carbon nanomaterial-based polymer composites. The present review covers important literature on different carbon nanomaterial-based PEMs reported during the last decade. The review emphasises the proton conductivity and methanol permeability of nanocomposite membranes with carbon nanotubes, graphene oxide and fullerene as additives, assessing critically the impact of each type of filler on those properties.
Keywords: carbon nanotubes; direct methanol fuel cell; graphene oxide; proton exchange membranes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













Similar articles
-
Potential carbon nanomaterials as additives for state-of-the-art Nafion electrolyte in proton-exchange membrane fuel cells: a concise review.RSC Adv. 2021 May 21;11(30):18351-18370. doi: 10.1039/d1ra00685a. eCollection 2021 May 19. RSC Adv. 2021. PMID: 35480954 Free PMC article. Review.
-
Modified Cellulose Proton-Exchange Membranes for Direct Methanol Fuel Cells.Polymers (Basel). 2023 Jan 27;15(3):659. doi: 10.3390/polym15030659. Polymers (Basel). 2023. PMID: 36771960 Free PMC article. Review.
-
Modifications on Promoting the Proton Conductivity of Polybenzimidazole-Based Polymer Electrolyte Membranes in Fuel Cells.Membranes (Basel). 2021 Oct 27;11(11):826. doi: 10.3390/membranes11110826. Membranes (Basel). 2021. PMID: 34832055 Free PMC article. Review.
-
A graphene oxide polymer brush based cross-linked nanocomposite proton exchange membrane for direct methanol fuel cells.RSC Adv. 2018 Apr 26;8(28):15740-15753. doi: 10.1039/c8ra01731j. eCollection 2018 Apr 23. RSC Adv. 2018. PMID: 35539468 Free PMC article.
-
SGO/SPES-based highly conducting polymer electrolyte membranes for fuel cell application.ACS Appl Mater Interfaces. 2014 Apr 23;6(8):5595-601. doi: 10.1021/am5000504. Epub 2014 Apr 15. ACS Appl Mater Interfaces. 2014. PMID: 24697540
Cited by
-
One-Step Microwave-Assisted Synthesis of PtNiCo/rGO Electrocatalysts with High Electrochemical Performance for Direct Methanol Fuel Cells.Nanomaterials (Basel). 2021 Aug 27;11(9):2206. doi: 10.3390/nano11092206. Nanomaterials (Basel). 2021. PMID: 34578522 Free PMC article.
-
Applications of Nanomaterials in Microbial Fuel Cells: A Review.Molecules. 2022 Nov 2;27(21):7483. doi: 10.3390/molecules27217483. Molecules. 2022. PMID: 36364309 Free PMC article. Review.
-
Multifunctional ternary ZnMgFe LDH as an efficient adsorbent for ceftriaxone sodium and antimicrobial agent: sustainability of adsorption waste as a catalyst for methanol electro-oxidation.RSC Adv. 2023 Sep 1;13(37):26069-26088. doi: 10.1039/d3ra03426g. eCollection 2023 Aug 29. RSC Adv. 2023. PMID: 37664207 Free PMC article.
-
Characterization of PBI/Graphene Oxide Composite Membranes for the SO2 Depolarized Electrolysis at High Temperature.Membranes (Basel). 2022 Jan 20;12(2):116. doi: 10.3390/membranes12020116. Membranes (Basel). 2022. PMID: 35207038 Free PMC article.
-
Potential carbon nanomaterials as additives for state-of-the-art Nafion electrolyte in proton-exchange membrane fuel cells: a concise review.RSC Adv. 2021 May 21;11(30):18351-18370. doi: 10.1039/d1ra00685a. eCollection 2021 May 19. RSC Adv. 2021. PMID: 35480954 Free PMC article. Review.
References
-
- McNicol B.D., Rand D.A.J., Williams K.R. Direct methanol-air fuel cells for road transportation. J. Power Sources. 1999;83:15–31. doi: 10.1016/S0378-7753(99)00244-X. - DOI
-
- Antonucci V., Shukla A.K., Arico A.S. An appraisal of electric automobile power sources. Renew. Sustain. Energy Rev. 2001;5:137–155.
-
- Kamarudin S.K., Achmad F., Daud W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrog. Energy. 2009;34:6902–6916. doi: 10.1016/j.ijhydene.2009.06.013. - DOI
-
- Samms S.R., Wasmus S., Savinell R.F. Thermal stability of Nafion® in simulated fuel cell environments. J. Electrochem. Soc. 1996;143:1498–1504. doi: 10.1149/1.1836669. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

