Migration of Small Ribosomal Subunits on the 5' Untranslated Regions of Capped Messenger RNA
- PMID: 31510048
- PMCID: PMC6769788
- DOI: 10.3390/ijms20184464
Migration of Small Ribosomal Subunits on the 5' Untranslated Regions of Capped Messenger RNA
Abstract
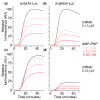
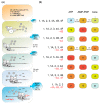
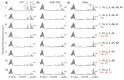
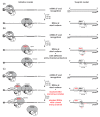
Several control mechanisms of eukaryotic gene expression target the initiation step of mRNA translation. The canonical translation initiation pathway begins with cap-dependent attachment of the small ribosomal subunit (SSU) to the messenger ribonucleic acid (mRNA) followed by an energy-dependent, sequential 'scanning' of the 5' untranslated regions (UTRs). Scanning through the 5'UTR requires the adenosine triphosphate (ATP)-dependent RNA helicase eukaryotic initiation factor (eIF) 4A and its efficiency contributes to the specific rate of protein synthesis. Thus, understanding the molecular details of the scanning mechanism remains a priority task for the field. Here, we studied the effects of inhibiting ATP-dependent translation and eIF4A in cell-free translation and reconstituted initiation reactions programmed with capped mRNAs featuring different 5'UTRs. An aptamer that blocks eIF4A in an inactive state away from mRNA inhibited translation of capped mRNA with the moderately structured β-globin sequences in the 5'UTR but not that of an mRNA with a poly(A) sequence as the 5'UTR. By contrast, the nonhydrolysable ATP analogue β,γ-imidoadenosine 5'-triphosphate (AMP-PNP) inhibited translation irrespective of the 5'UTR sequence, suggesting that complexes that contain ATP-binding proteins in their ATP-bound form can obstruct and/or actively block progression of ribosome recruitment and/or scanning on mRNA. Further, using primer extension inhibition to locate SSUs on mRNA ('toeprinting'), we identify an SSU complex which inhibits primer extension approximately eight nucleotides upstream from the usual toeprinting stop generated by SSUs positioned over the start codon. This '-8 nt toeprint' was seen with mRNA 5'UTRs of different length, sequence and structure potential. Importantly, the '-8 nt toeprint' was strongly stimulated by the presence of the cap on the mRNA, as well as the presence of eIFs 4F, 4A/4B and ATP, implying active scanning. We assembled cell-free translation reactions with capped mRNA featuring an extended 5'UTR and used cycloheximide to arrest elongating ribosomes at the start codon. Impeding scanning through the 5'UTR in this system with elevated magnesium and AMP-PNP (similar to the toeprinting conditions), we visualised assemblies consisting of several SSUs together with one full ribosome by electron microscopy, suggesting direct detection of scanning intermediates. Collectively, our data provide additional biochemical, molecular and physical evidence to underpin the scanning model of translation initiation in eukaryotes.
Keywords: 40S ribosomal subunit; 5′ UTR; 5′UTR; SSU; cap-dependent initiation; eIF4A; eIF4F; eukaryotes; gene expression control; mRNA translation; ribosomal scanning; translation initiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Efficient translation initiation directed by the 900-nucleotide-long and GC-rich 5' untranslated region of the human retrotransposon LINE-1 mRNA is strictly cap dependent rather than internal ribosome entry site mediated.Mol Cell Biol. 2007 Jul;27(13):4685-97. doi: 10.1128/MCB.02138-06. Epub 2007 Apr 30. Mol Cell Biol. 2007. PMID: 17470553 Free PMC article.
-
Mechanistic differences in eukaryotic initiation factor requirements for eIF4GI-driven cap-independent translation of structured mRNAs.J Biol Chem. 2024 Nov;300(11):107866. doi: 10.1016/j.jbc.2024.107866. Epub 2024 Oct 9. J Biol Chem. 2024. PMID: 39384039 Free PMC article.
-
The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection.Genes Dev. 2002 Nov 15;16(22):2906-22. doi: 10.1101/gad.1020902. Genes Dev. 2002. PMID: 12435632 Free PMC article.
-
Cap-dependent, scanning-free translation initiation mechanisms.Biochim Biophys Acta. 2015 Nov;1849(11):1313-8. doi: 10.1016/j.bbagrm.2015.09.006. Epub 2015 Sep 14. Biochim Biophys Acta. 2015. PMID: 26381322 Review.
-
More than just scanning: the importance of cap-independent mRNA translation initiation for cellular stress response and cancer.Cell Mol Life Sci. 2017 May;74(9):1659-1680. doi: 10.1007/s00018-016-2428-2. Epub 2016 Dec 2. Cell Mol Life Sci. 2017. PMID: 27913822 Free PMC article. Review.
Cited by
-
eIF4A1-dependent mRNAs employ purine-rich 5'UTR sequences to activate localised eIF4A1-unwinding through eIF4A1-multimerisation to facilitate translation.Nucleic Acids Res. 2023 Feb 28;51(4):1859-1879. doi: 10.1093/nar/gkad030. Nucleic Acids Res. 2023. PMID: 36727461 Free PMC article.
-
Roles of the CCR4-Not complex in translation and dynamics of co-translation events.Wiley Interdiscip Rev RNA. 2023 Nov 27;15(1):e1827. doi: 10.1002/wrna.1827. Online ahead of print. Wiley Interdiscip Rev RNA. 2023. PMID: 38009591 Free PMC article. Review.
-
The molecular basis of translation initiation and its regulation in eukaryotes.Nat Rev Mol Cell Biol. 2024 Mar;25(3):168-186. doi: 10.1038/s41580-023-00624-9. Epub 2023 Dec 5. Nat Rev Mol Cell Biol. 2024. PMID: 38052923 Review.
-
How Many Messenger RNAs Can Be Translated by the START Mechanism?Int J Mol Sci. 2020 Nov 8;21(21):8373. doi: 10.3390/ijms21218373. Int J Mol Sci. 2020. PMID: 33171614 Free PMC article.
-
ALKBH3 partner ASCC3 mediates P-body formation and selective clearance of MMS-induced 1-methyladenosine and 3-methylcytosine from mRNA.J Transl Med. 2021 Jul 3;19(1):287. doi: 10.1186/s12967-021-02948-6. J Transl Med. 2021. PMID: 34217309 Free PMC article.
References
-
- Kozak M., Shatkin A.J. Migration of 40 S ribosomal subunits on messenger RNA in the presence of edeine. J. Biol. Chem. 1978;253:6568–6577. - PubMed
-
- Pisarev A.V., Kolupaeva V.G., Pisareva V.P., Merrick W.C., Hellen C.U., Pestova T.V. Specific functional interactions of nucleotides at key -3 and +4 positions flanking the initiation codon with components of the mammalian 48S translation initiation complex. Genes. Dev. 2006;20:624–636. doi: 10.1101/gad.1397906. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

