Characterizing Tyrosinase Modulators from the Roots of Angelica keiskei Using Tyrosinase Inhibition Assay and UPLC-MS/MS as the Combinatorial Novel Approach
- PMID: 31510069
- PMCID: PMC6767278
- DOI: 10.3390/molecules24183297
Characterizing Tyrosinase Modulators from the Roots of Angelica keiskei Using Tyrosinase Inhibition Assay and UPLC-MS/MS as the Combinatorial Novel Approach
Abstract
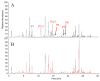
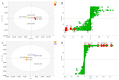
In this study, an in vitro tyrosinase inhibition assay in combination with ultra performance liquid chromatography-orbitrap mass spectrometry (UPLC-orbitrap-MS) was developed for the rapid screening and identification of tyrosinase modulators from roots of Angelica keiskei. Of the 15 candidates considered, nine chalcones, xanthoangelols (1), B (2), D (3), E (4), G (5), H (6), 4-hydroxyderricin (7), xanthokeismin B (8) and (2E)-1-[4-hydroxy-2-(2-hydroxy-2-propanyl)-2,3-dihydro-1-benzofuran-7-yl]-3-(4-hydroxyphenyl)-2-propen-1-one (9), five coumarins, umbelliferone (10), selinidin (11), isopimpinellin (12), phellopterin (13) and xanthyletin (14), and one other compound, ashitabaol A (15), were distinguished between the test samples and the controls with statistical significance, and the structure of each compound was determined by comparing with in-house standards and the literature. Among these, six compounds, xanthoangelol (1), xanthoangelol D (3), xanthoangelol H (6), 4-hydroxyderricin (7), laserpitin (16) and isolaserpitin (17), were isolated from roots of A. keiskei. Of the compounds isolated, compounds 1, 7 and 16 were subjected to tyrosinase inhibitory assay, and the IC50 values were 15.87 ± 1.21, 60.14 ± 2.29 and >100 μM, respectively. The present study indicated that the combination of in vitro tyrosinase inhibition assay coupled with UPLC-MS/MS could be widely applied to the rapid screening of active substances from various natural resources.
Keywords: Angelica keiskei; UPLC-MS/MS; chalcone; coumarins; tyrosinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Bioassay-guided isolation and identification of anti-platelet-active compounds from the root of Ashitaba (Angelica keiskei Koidz.).Nat Prod Res. 2014;28(24):2312-6. doi: 10.1080/14786419.2014.931389. Epub 2014 Jul 14. Nat Prod Res. 2014. PMID: 25019280
-
PTP1B inhibitors from stems of Angelica keiskei (Ashitaba).Bioorg Med Chem Lett. 2015;25(10):2028-32. doi: 10.1016/j.bmcl.2015.04.003. Epub 2015 Apr 4. Bioorg Med Chem Lett. 2015. PMID: 25891102
-
Chalcones from Angelica keiskei: Evaluation of Their Heat Shock Protein Inducing Activities.J Nat Prod. 2015 Oct 23;78(10):2481-7. doi: 10.1021/acs.jnatprod.5b00633. Epub 2015 Oct 2. J Nat Prod. 2015. PMID: 26431394
-
A comprehensive review on tyrosinase inhibitors.J Enzyme Inhib Med Chem. 2019 Dec;34(1):279-309. doi: 10.1080/14756366.2018.1545767. J Enzyme Inhib Med Chem. 2019. PMID: 30734608 Free PMC article. Review.
-
Toxicological assessment of Ashitaba Chalcone.Food Chem Toxicol. 2015 Mar;77:111-9. doi: 10.1016/j.fct.2014.12.021. Epub 2015 Jan 7. Food Chem Toxicol. 2015. PMID: 25576957 Review.
Cited by
-
Food and Drug Analysis.Molecules. 2020 May 21;25(10):2403. doi: 10.3390/molecules25102403. Molecules. 2020. PMID: 32455805 Free PMC article.
-
Plant Extracts as Skin Care and Therapeutic Agents.Int J Mol Sci. 2023 Oct 22;24(20):15444. doi: 10.3390/ijms242015444. Int J Mol Sci. 2023. PMID: 37895122 Free PMC article. Review.
-
A Review on the Morphology, Cultivation, Identification, Phytochemistry, and Pharmacology of Kitagawia praeruptora (Dunn) Pimenov.Molecules. 2023 Dec 18;28(24):8153. doi: 10.3390/molecules28248153. Molecules. 2023. PMID: 38138641 Free PMC article. Review.
-
Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021.Molecules. 2021 Dec 13;26(24):7546. doi: 10.3390/molecules26247546. Molecules. 2021. PMID: 34946631 Free PMC article. Review.
-
Structure-Based Virtual Screening of Furan-1,3,4-Oxadiazole Tethered N-phenylacetamide Derivatives as Novel Class of hTYR and hTYRP1 Inhibitors.Pharmaceuticals (Basel). 2023 Feb 23;16(3):344. doi: 10.3390/ph16030344. Pharmaceuticals (Basel). 2023. PMID: 36986444 Free PMC article.
References
-
- Picardo M., Maresca V., Eibenschutz L., De Bernardo C., Rinaldi R., Grammatico P. Correlation Between Antioxidants and Phototypes in Melanocytes Cultures. A Possible Link of Physiologic and Pathologic Relevance. J. Investig. Dermatol. 1999;113:424–425. - PubMed
-
- Pillaiyar T., Namasivayam V., Manickam M., Jung S.-H. Inhibitors of melanogenesis: An updated review. J. Med. Chem. 2018;61:7395–7418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

