Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia
- PMID: 31511239
- PMCID: PMC6923664
- DOI: 10.1182/blood.2019000069
Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia
Abstract
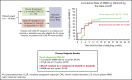
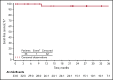
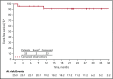
Chronic myeloid leukemia (CML) is rare in children and accounts for ≤15% of all myeloid leukemia cases. When we initiated this study with nilotinib, imatinib was the only tyrosine kinase inhibitor indicated for pediatric patients with Philadelphia chromosome-positive (Ph+) CML in chronic phase (CP); alternative treatment options were needed, particularly for patients who developed resistance or intolerance (R/I) to imatinib. This phase 2 study enrolled pediatric patients with either Ph+ CML-CP R/I to imatinib or dasatinib or newly diagnosed Ph+ CML-CP. Data presented are from analyses with minimum follow-up of up to 24 cycles (1 cycle is 28 days). Fifty-nine patients with Ph+ CML-CP were enrolled, and 58 were treated (R/I, n = 33; newly diagnosed, n = 25). Major molecular response (MMR) rate at cycle 6 in the R/I cohort was 39.4% (primary end point); 57.6% of patients achieved or maintained MMR and 81.8% achieved or maintained complete cytogenetic response (CCyR) by 24 cycles. In patients with newly diagnosed disease, rates of MMR by cycle 12 and CCyR at cycle 12 were 64.0% each (primary end points); by cycle 24, cumulative MMR and CCyR rates were 68.0% and 84.0%, respectively. The safety profile of nilotinib in pediatric patients was generally comparable with the known safety profile in adults, although cardiovascular events were not observed in this study, and hepatic laboratory abnormalities were more frequent; no new safety signals were identified. In summary, nilotinib demonstrated efficacy and a manageable safety profile in pediatric patients with Ph+ CML-CP. This trial was registered at www.clinicaltrials.gov as #NCT01844765.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: N.H. and C.M.Z. received honoraria and served in a consulting or advisory role for Novartis. C.R. received honoraria, served in a consulting or advisory role, and received compensation for travel from Jazz Pharmaceuticals, Amgen, and Shire. C.D. served in a consulting or advisory role for Novartis. Z.K. received research grants from Novartis. P.A., A.A., S.Q., F.H.-P., and S.H. are employees of Novartis. S.H. owns stock in Novartis. The remaining authors declare no competing financial interests.
Figures
References
-
- Hijiya N, Millot F, Suttorp M. Chronic myeloid leukemia in children: clinical findings, management, and unanswered questions. Pediatr Clin North Am. 2015;62(1):107-119. - PubMed
-
- Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:368-376. - PubMed
-
- Ries LAG, Smith MA, Gurney JG, et al. . Cancer incidence and survival among children and adolescents. United States SEER Program 1975-1995. Bethesda, MD: 1999. NIH publication number 99-4649.
-
- Novartis Pharmaceuticals Corporation Novartis drug Tasigna (nilotinib) secures EU approval for first and second-line treatment of Ph+ CML-CP in children. https://www.novartis.com/news/media-releases/novartis-drug-tasignar-nilo.... Accessed 20 June 2019.
-
- U.S. Food and Drug Administration FDA approves dasatinib for pediatric patients with CML. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro.... Accessed 20 June 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

