Casein kinase 1α decreases β-catenin levels at adherens junctions to facilitate wound closure in Drosophila larvae
- PMID: 31511254
- PMCID: PMC6826034
- DOI: 10.1242/dev.175133
Casein kinase 1α decreases β-catenin levels at adherens junctions to facilitate wound closure in Drosophila larvae
Abstract
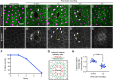
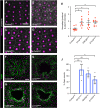
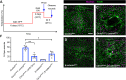
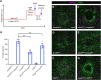
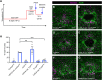
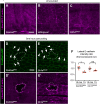
Skin wound repair is essential to restore barrier function and prevent infection after tissue damage. Wound-edge epidermal cells migrate as a sheet to close the wound. However, it is still unclear how cell-cell junctions are regulated during wound closure (WC). To study this, we examined adherens junctions during WC in Drosophila larvae. β-Catenin is reduced at the lateral cell-cell junctions of wound-edge epidermal cells in the early healing stages. Destruction complex components, including Ck1α, GSK3β and β-TrCP, suppress β-catenin levels in the larval epidermis. Tissue-specific RNAi targeting these genes also caused severe WC defects. The Ck1αRNAi -induced WC defect is related to adherens junctions because loss of either β-catenin or E-cadherin significantly rescued this WC defect. In contrast, TCFRNAi does not rescue the Ck1αRNAi -induced WC defect, suggesting that Wnt signaling is not related to this defect. Direct overexpression of β-catenin recapitulates most of the features of Ck1α reduction during wounding. Finally, loss of Ck1α also blocked junctional E-cadherin reduction around the wound. Our results suggest that Ck1α and the destruction complex locally regulate cell adhesion to facilitate efficient wound repair.
Keywords: Adherens junctions; Casein kinase 1α; Drosophila; Epithelium; Wound repair; β-Catenin.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Splice variants of CK1α and CK1α-like: Comparative analysis of subcellular localization, kinase activity, and function in the Wnt signaling pathway.J Biol Chem. 2024 Jul;300(7):107407. doi: 10.1016/j.jbc.2024.107407. Epub 2024 May 23. J Biol Chem. 2024. PMID: 38796065 Free PMC article.
-
Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions.PLoS One. 2010 Nov 30;5(11):e14127. doi: 10.1371/journal.pone.0014127. PLoS One. 2010. PMID: 21152425 Free PMC article.
-
Stromal cell derived factor-1α promotes C-Kit+ cardiac stem/progenitor cell quiescence through casein kinase 1α and GSK3β.Stem Cells. 2014 Feb;32(2):487-99. doi: 10.1002/stem.1534. Stem Cells. 2014. PMID: 24038789 Free PMC article.
-
Suppression of casein kinase 1alpha in melanoma cells induces a switch in beta-catenin signaling to promote metastasis.Cancer Res. 2010 Sep 1;70(17):6999-7009. doi: 10.1158/0008-5472.CAN-10-0645. Epub 2010 Aug 10. Cancer Res. 2010. PMID: 20699366
-
MDMX inhibits casein kinase 1α activity and stimulates Wnt signaling.EMBO J. 2020 Jul 15;39(14):e104410. doi: 10.15252/embj.2020104410. Epub 2020 Jun 8. EMBO J. 2020. PMID: 32511789 Free PMC article.
Cited by
-
Wound Healing Insights from Flies and Fish.Cold Spring Harb Perspect Biol. 2022 Nov 1;14(11):a041217. doi: 10.1101/cshperspect.a041217. Cold Spring Harb Perspect Biol. 2022. PMID: 35817511 Free PMC article. Review.
-
Phosphorylation of Yun is required for stem cell proliferation and tumorigenesis.Cell Prolif. 2022 May;55(5):e13230. doi: 10.1111/cpr.13230. Epub 2022 Apr 18. Cell Prolif. 2022. PMID: 35437864 Free PMC article.
-
Paxillin tunes the relationship between cell-matrix and cell-cell adhesions to regulate stiffness-dependent dentinogenesis.Regen Biomater. 2022 Dec 10;10:rbac100. doi: 10.1093/rb/rbac100. eCollection 2023. Regen Biomater. 2022. PMID: 36683745 Free PMC article.
References
-
- Brand A. H. and Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

