Unexpected roles for ADH1 and SORD in catalyzing the final step of erythritol biosynthesis
- PMID: 31511322
- PMCID: PMC6827307
- DOI: 10.1074/jbc.RA119.009049
Unexpected roles for ADH1 and SORD in catalyzing the final step of erythritol biosynthesis
Abstract
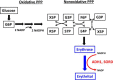
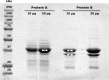
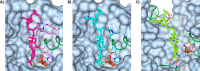
The low-calorie sweetener erythritol is endogenously produced from glucose through the pentose phosphate pathway in humans. Erythritol is of medical interest because elevated plasma levels of this polyol are predictive for visceral adiposity gain and development of type 2 diabetes. However, the mechanisms behind these associations remain unknown because the erythritol biosynthesis pathway, particularly the enzyme catalyzing the final step of erythritol synthesis (reduction of erythrose to erythritol), is not characterized. In this study, we purified two enzymes from rabbit liver capable of catalyzing the conversion of erythrose to erythritol: alcohol dehydrogenase 1 (ADH1) and sorbitol dehydrogenase (SORD). Both recombinant human ADH1 and SORD reduce erythrose to erythritol, using NADPH as a co-factor, and cell culture studies indicate that this activity is primarily NADPH-dependent. We found that ADH1 variants vary markedly in both their affinity for erythrose and their catalytic capacity (turnover number). Interestingly, the recombinant protein produced from the ADH1B2 variant, common in Asian populations, is not active when NADPH is used as a co-factor in vitro We also confirmed SORD contributes to intracellular erythritol production in human A549 lung cancer cells, where ADH1 is minimally expressed. In summary, human ADH1 and SORD catalyze the conversion of erythrose to erythritol, pointing to novel roles for two dehydrogenase proteins in human glucose metabolism that may contribute to individual responses to diet. Proteomics data are available via ProteomeXchange with identifier PXD015178.
Keywords: alcohol dehydrogenase (ADH); biomarker; enzyme catalysis; enzyme kinetics; erythritol; glucose metabolism; sorbitol dehydrogenase.
© 2019 Schlicker et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Hootman K. C., Trezzi J.-P., Kraemer L., Kraemer L., Burnwell L. S., Dong X., Guertin K. A., Jaeger C., Stober P. J., Hiller K., and Cassano P. A. (2017) Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc. Natl. Acad. Sci. U.S.A. 114, E4233–E4240 10.1073/pnas.1620079114 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

