Prion protein modulates endothelial to mesenchyme-like transition in trabecular meshwork cells: Implications for primary open angle glaucoma
- PMID: 31511544
- PMCID: PMC6739364
- DOI: 10.1038/s41598-019-49482-6
Prion protein modulates endothelial to mesenchyme-like transition in trabecular meshwork cells: Implications for primary open angle glaucoma
Abstract
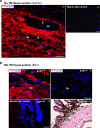
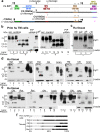
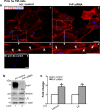
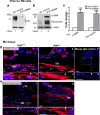
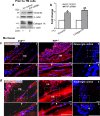
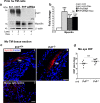
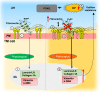
Endothelial-to-mesenchyme-like transition (Endo-MT) of trabecular meshwork (TM) cells is known to be associated with primary open angle glaucoma (POAG). Here, we investigated whether the prion protein (PrPC), a neuronal protein known to modulate epithelial-to-mesenchymal transition in a variety of cell types, is expressed in the TM, and plays a similar role at this site. Using a combination of primary human TM cells and human, bovine, and PrP-knock-out (PrP-/-) mouse models, we demonstrate that PrPC is expressed in the TM of all three species, including endothelial cells lining the Schlemm's canal. Silencing of PrPC in primary human TM cells induces aggregation of β1-integrin and upregulation of α-smooth muscle actin, fibronectin, collagen 1A, vimentin, and laminin, suggestive of transition to a mesenchyme-like phenotype. Remarkably, intraocular pressure is significantly elevated in PrP-/- mice relative to wild-type controls, suggesting reduced pliability of the extracellular matrix and increased resistance to aqueous outflow in the absence of PrPC. Since PrPC is cleaved by members of the disintegrin and matrix-metalloprotease family that are increased in the aqueous humor of POAG arising from a variety of conditions, it is likely that concomitant cleavage of PrPC exaggerates and confounds the pathology by inducing Endo-MT-like changes in the TM.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
α-Synuclein modulates fibronectin expression in the trabecular meshwork independent of TGFβ2.Exp Eye Res. 2023 Jan;226:109351. doi: 10.1016/j.exer.2022.109351. Epub 2022 Dec 17. Exp Eye Res. 2023. PMID: 36539052 Free PMC article.
-
TGFβ2 alters segmental outflow and ECM ultrastructure in the trabecular meshwork.Exp Eye Res. 2025 Jun;255:110377. doi: 10.1016/j.exer.2025.110377. Epub 2025 Apr 10. Exp Eye Res. 2025. PMID: 40216065
-
Age-Related Dysregulation of α5β1 and αvβ3 Integrin Activity Alters Contractile Properties of Trabecular Meshwork Cells.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):31. doi: 10.1167/iovs.66.6.31. Invest Ophthalmol Vis Sci. 2025. PMID: 40488712 Free PMC article.
-
Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension.Cochrane Database Syst Rev. 2022 Jun 10;6(6):CD013817. doi: 10.1002/14651858.CD013817.pub2. Cochrane Database Syst Rev. 2022. PMID: 35686679 Free PMC article.
-
Role of Glucocorticoids and Glucocorticoid Receptors in Glaucoma Pathogenesis.Cells. 2023 Oct 14;12(20):2452. doi: 10.3390/cells12202452. Cells. 2023. PMID: 37887296 Free PMC article. Review.
Cited by
-
Prions and prion diseases: Insights from the eye.Exp Eye Res. 2020 Oct;199:108200. doi: 10.1016/j.exer.2020.108200. Epub 2020 Aug 25. Exp Eye Res. 2020. PMID: 32858007 Free PMC article. Review.
-
α-Synuclein modulates fibronectin expression in the trabecular meshwork independent of TGFβ2.Exp Eye Res. 2023 Jan;226:109351. doi: 10.1016/j.exer.2022.109351. Epub 2022 Dec 17. Exp Eye Res. 2023. PMID: 36539052 Free PMC article.
-
The role of Vitamin D3 in ocular fibrosis and its therapeutic potential for the glaucomatous trabecular meshwork.Front Ophthalmol (Lausanne). 2022 Aug 1;2:897118. doi: 10.3389/fopht.2022.897118. eCollection 2022. Front Ophthalmol (Lausanne). 2022. PMID: 38983544 Free PMC article. Review.
-
Tissue-Engineered Models for Glaucoma Research.Micromachines (Basel). 2020 Jun 24;11(6):612. doi: 10.3390/mi11060612. Micromachines (Basel). 2020. PMID: 32599818 Free PMC article. Review.
-
Upregulation of Local Hepcidin Contributes to Iron Accumulation in Alzheimer's Disease Brains.J Alzheimers Dis. 2021;82(4):1487-1497. doi: 10.3233/JAD-210221. J Alzheimers Dis. 2021. PMID: 34180415 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

