Ixodes scapularis salivary gland microRNAs are differentially expressed during Powassan virus transmission
- PMID: 31511580
- PMCID: PMC6739385
- DOI: 10.1038/s41598-019-49572-5
Ixodes scapularis salivary gland microRNAs are differentially expressed during Powassan virus transmission
Abstract
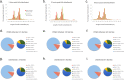
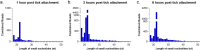
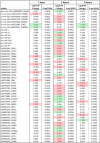
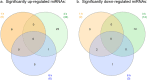
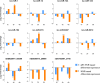
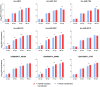
Successful tick feeding is facilitated by an assortment of pharmacologically-active factors in tick saliva that create an immunologically privileged micro-environment in the host's skin. Through a process known as saliva-assisted transmission, bioactive tick salivary factors modulate the host environment, promoting transmission and establishment of a tick-borne pathogen. This phenomenon was previously demonstrated for Powassan virus (POWV), a North American tick-borne flavivirus that is the causative agent of a severe neuroinvasive disease in humans. Here, we sought to characterize the Ixodes scapularis salivary gland microRNAs (miRNAs) expressed during the earliest period of POWV transmission to a mammalian host. POWV-infected and uninfected I. scapularis females were fed on naïve mice for 1, 3, and 6 hours, and Illumina next generation sequencing was used to characterize the salivary gland miRNA expression profiles of POWV-infected versus uninfected ticks. 379 salivary miRNAs were detected, of which 338 are reported here as putative novel I. scapularis miRNAs. 35 salivary gland miRNAs were significantly up-regulated and 17 miRNAs were significantly down-regulated in response to POWV infection. To investigate the potential role of salivary gland miRNAs in POWV replication in-vitro, we transfected miRNA inhibitors into VeroE6 cells to profile temporal POWV replication in mammalian cells. Together, the small RNA sequencing data and the in vitro miRNA inhibition assay suggest that the differentially expressed tick salivary miRNAs could act in regulating POWV replication in host tissues.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Pathogenicity and virulence of Powassan virus.Virulence. 2025 Dec;16(1):2523887. doi: 10.1080/21505594.2025.2523887. Epub 2025 Jun 26. Virulence. 2025. PMID: 40545598 Free PMC article. Review.
-
Tick Saliva Enhances Powassan Virus Transmission to the Host, Influencing Its Dissemination and the Course of Disease.J Virol. 2015 Aug;89(15):7852-60. doi: 10.1128/JVI.01056-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995246 Free PMC article.
-
Interspecies co-feeding transmission of Powassan virus between a native tick, Ixodes scapularis, and the invasive East Asian tick, Haemaphysalis longicornis.Parasit Vectors. 2024 Jun 15;17(1):259. doi: 10.1186/s13071-024-06335-0. Parasit Vectors. 2024. PMID: 38879603 Free PMC article.
-
Differential salivary gland transcript expression profile in Ixodes scapularis nymphs upon feeding or flavivirus infection.Ticks Tick Borne Dis. 2012 Feb;3(1):18-26. doi: 10.1016/j.ttbdis.2011.09.003. Epub 2012 Jan 2. Ticks Tick Borne Dis. 2012. PMID: 22309855 Free PMC article.
-
Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America.Vector Borne Zoonotic Dis. 2017 Jul;17(7):453-462. doi: 10.1089/vbz.2017.2110. Epub 2017 May 12. Vector Borne Zoonotic Dis. 2017. PMID: 28498740 Free PMC article. Review.
Cited by
-
Pathogenicity and virulence of Powassan virus.Virulence. 2025 Dec;16(1):2523887. doi: 10.1080/21505594.2025.2523887. Epub 2025 Jun 26. Virulence. 2025. PMID: 40545598 Free PMC article. Review.
-
Induced Transient Immune Tolerance in Ticks and Vertebrate Host: A Keystone of Tick-Borne Diseases?Front Immunol. 2021 Feb 12;12:625993. doi: 10.3389/fimmu.2021.625993. eCollection 2021. Front Immunol. 2021. PMID: 33643313 Free PMC article. Review.
-
Changing the Recipe: Pathogen Directed Changes in Tick Saliva Components.Int J Environ Res Public Health. 2021 Feb 12;18(4):1806. doi: 10.3390/ijerph18041806. Int J Environ Res Public Health. 2021. PMID: 33673273 Free PMC article. Review.
-
An Insight Into the microRNA Profile of the Ectoparasitic Mite Varroa destructor (Acari: Varroidae), the Primary Vector of Honey Bee Deformed Wing Virus.Front Cell Infect Microbiol. 2022 Mar 16;12:847000. doi: 10.3389/fcimb.2022.847000. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35372101 Free PMC article.
-
Host Immune Responses to Salivary Components - A Critical Facet of Tick-Host Interactions.Front Cell Infect Microbiol. 2022 Mar 16;12:809052. doi: 10.3389/fcimb.2022.809052. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35372098 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

